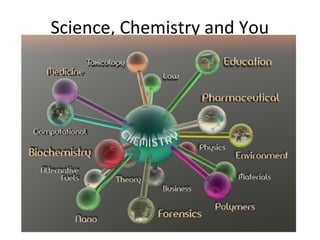
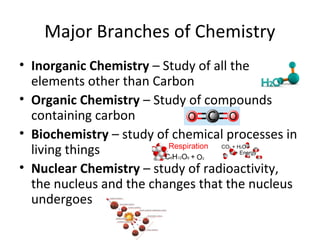
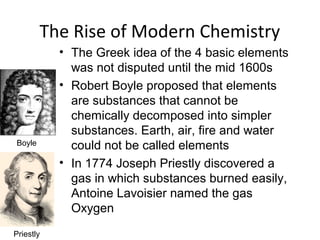
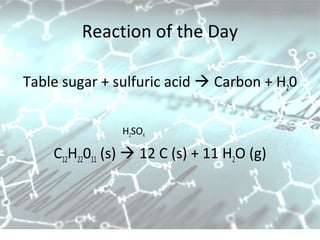
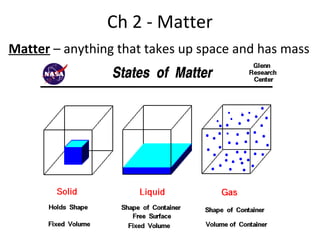
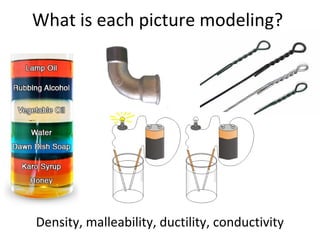
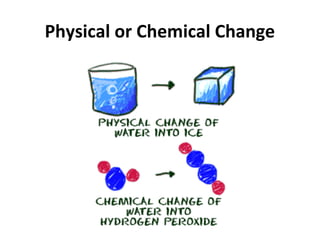
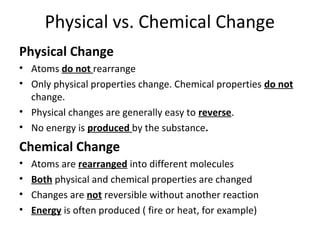
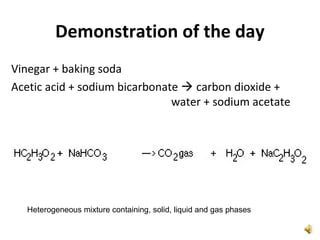
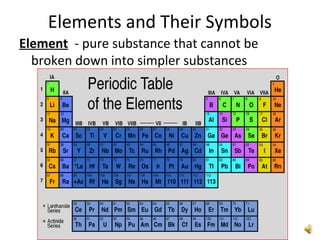
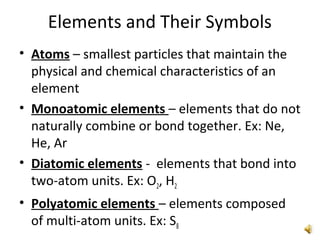
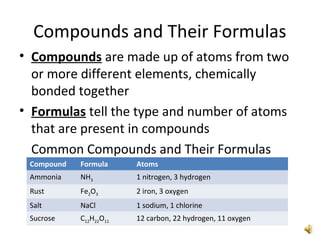
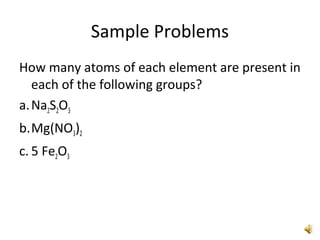
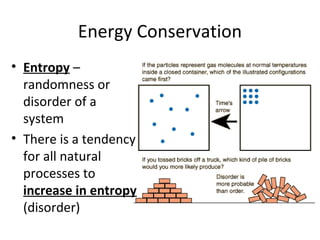
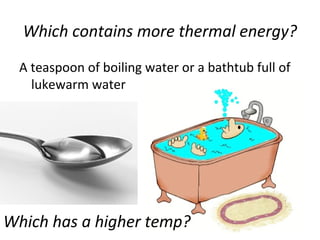
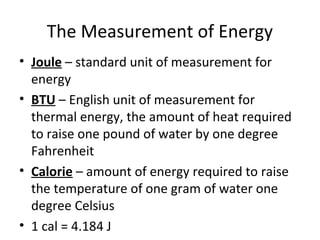
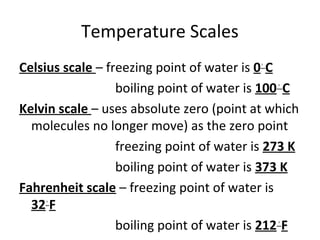
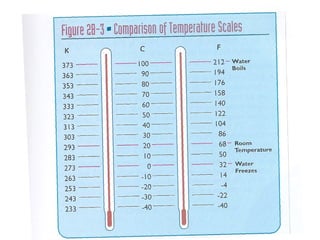
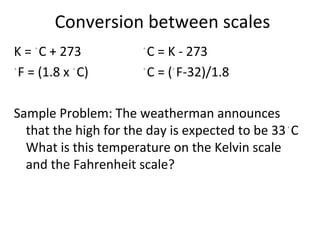
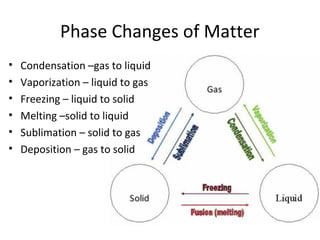
This document provides an overview of chemistry concepts including the definition of chemistry, major branches of chemistry, early theories of matter, and important figures in the development of modern chemistry such as Aristotle, Democritus, Boyle, Priestley, and Dalton. It also discusses the classification of matter as elements, compounds, and mixtures. Key chemistry concepts like physical and chemical properties, physical and chemical changes, energy, heat, and phase changes are introduced.