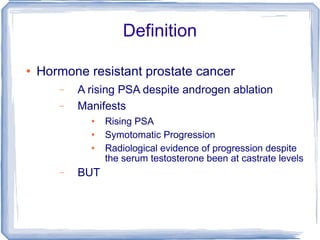
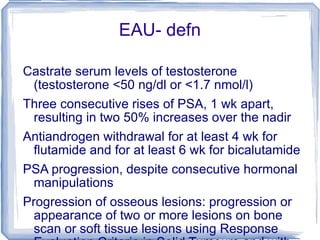
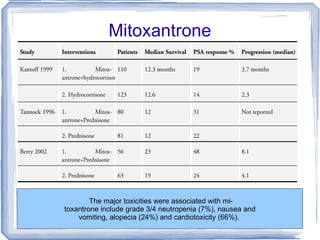
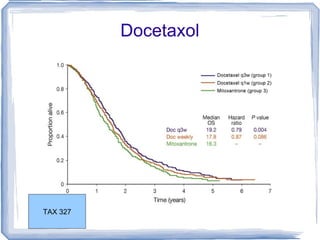
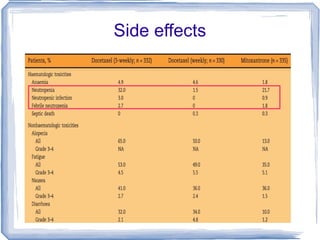
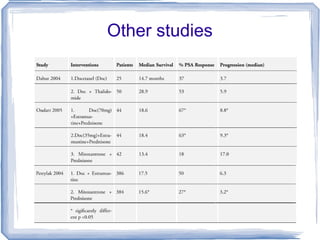
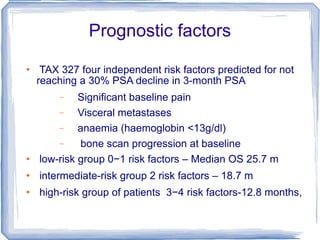
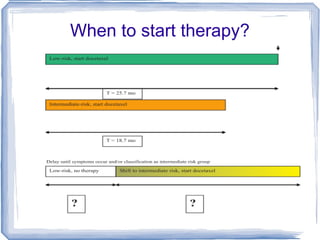
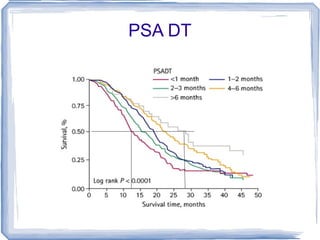
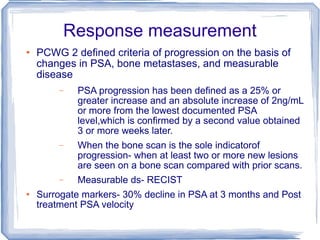
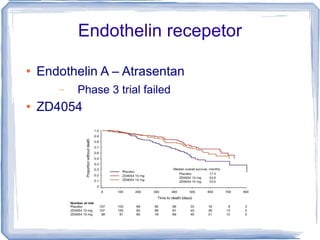
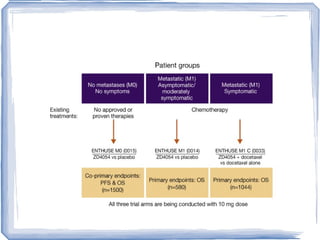
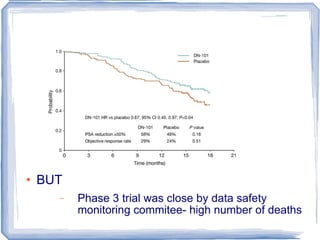
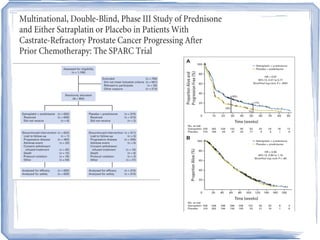
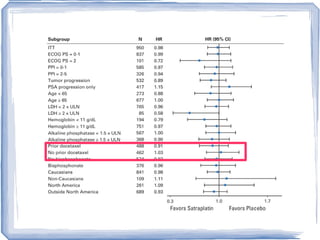
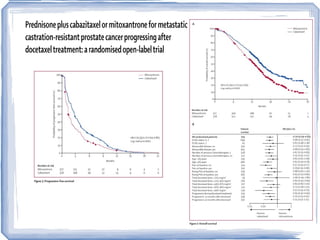
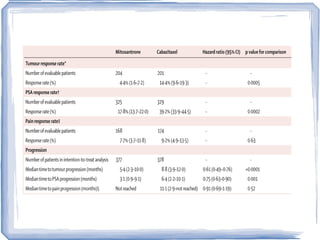
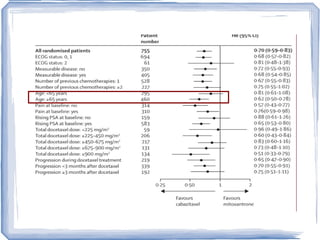
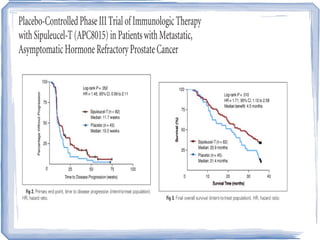
Chemotherapy can be used to treat hormone-resistant prostate cancer (HRPC) to help palliate symptoms and provide a survival benefit. Docetaxel plus prednisone was established as the standard first-line treatment based on results from the TAX 327 trial showing a median overall survival of around 18 months. Several prognostic factors can help predict survival outcomes on chemotherapy. For patients who progress after first-line docetaxel treatment, metronomic cyclophosphamide with prednisone shows promise as a well-tolerated second-line option based on early clinical trials. Ongoing research continues to evaluate new agents for first- and second-line HRPC.