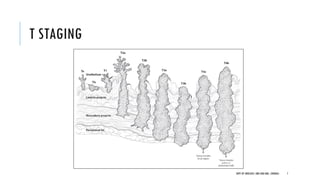
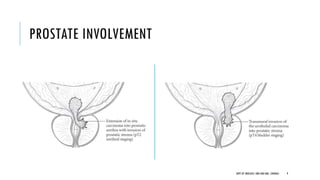
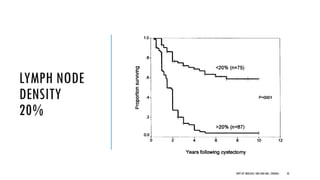
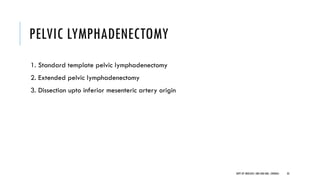
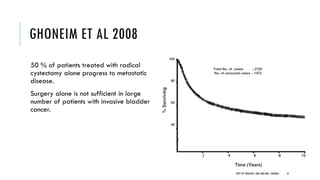
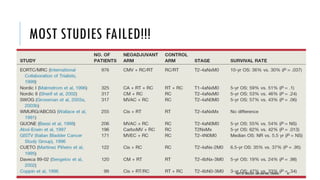
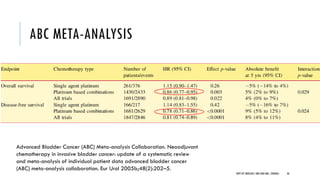
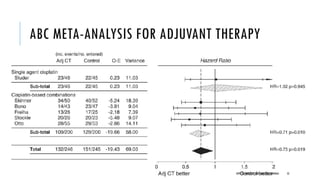
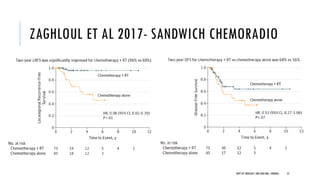
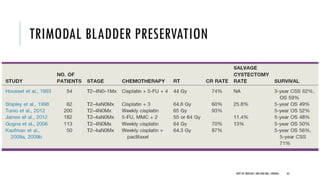
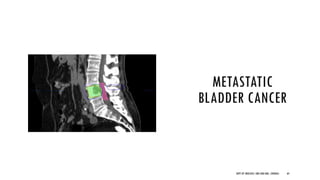
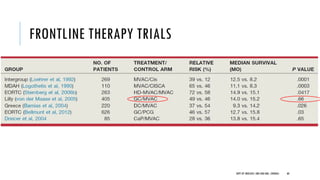
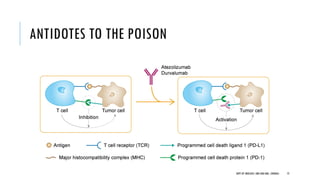
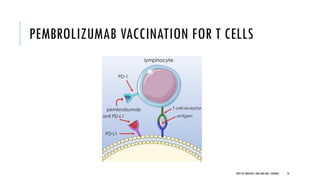
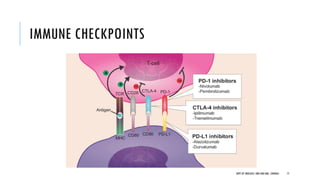
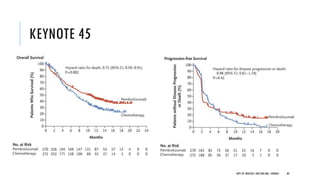
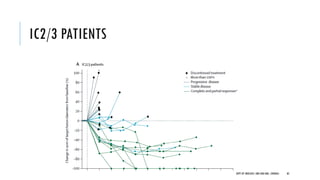
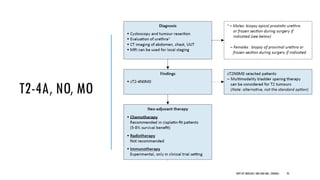
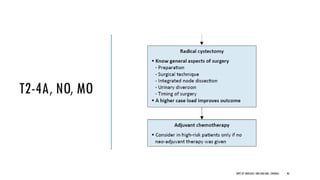
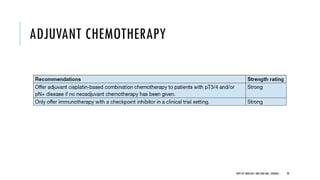
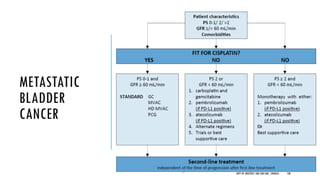
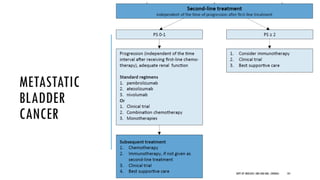
This document discusses muscle invasive bladder cancer (MIBC) and metastatic bladder cancer. It covers topics such as how MIBC is diagnosed, staging of MIBC using the TNM system, treatment with radical cystectomy and pelvic lymph node dissection, and use of neoadjuvant and adjuvant chemotherapy. It also discusses criteria for bladder preservation approaches and standards of care for treating metastatic bladder cancer with cisplatin-based chemotherapy.