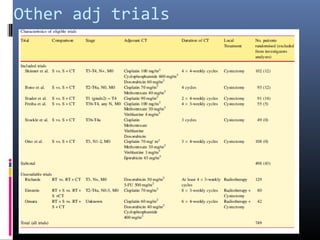
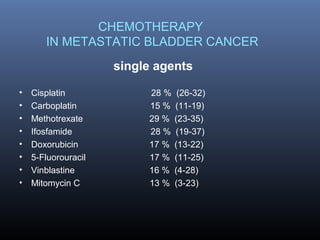
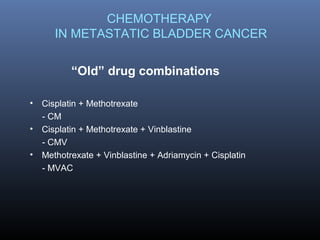
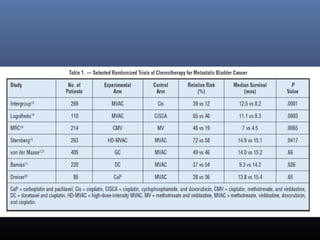
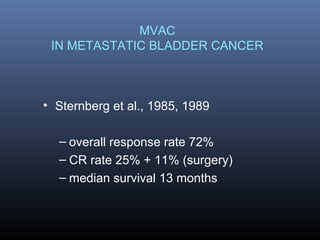
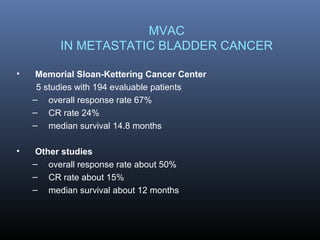
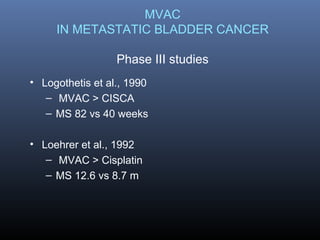
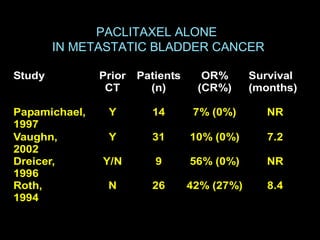
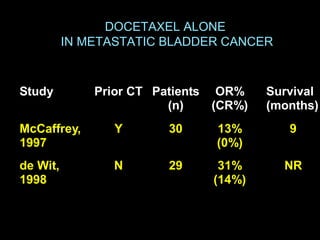
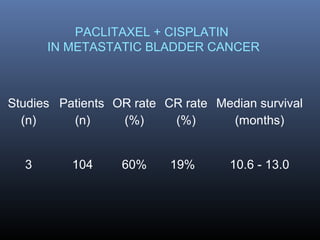
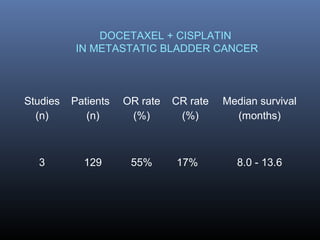
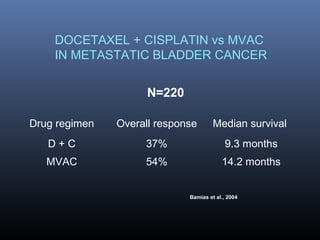
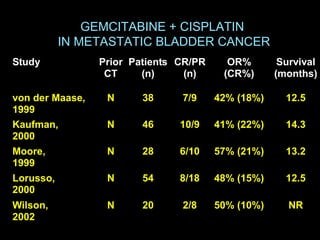
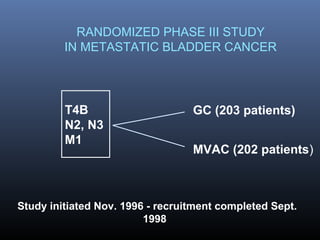
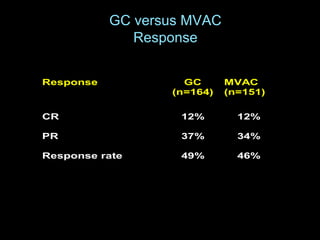
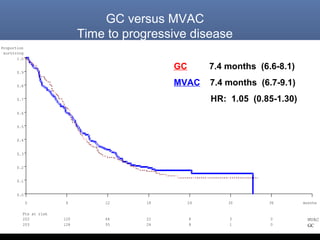
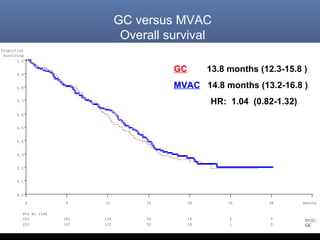
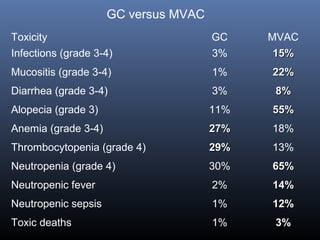
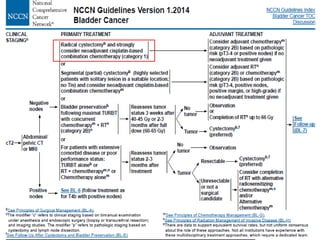
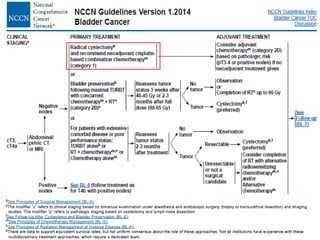
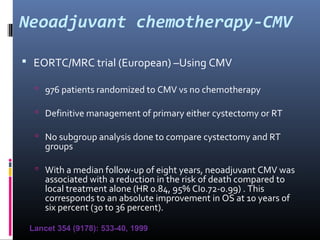
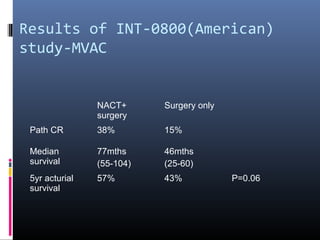
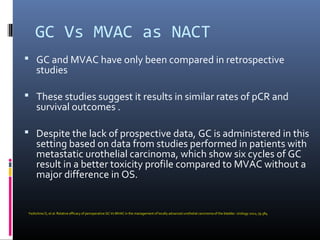
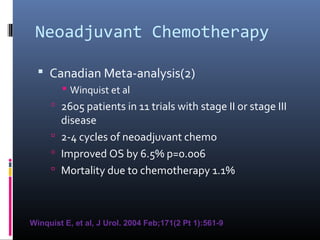
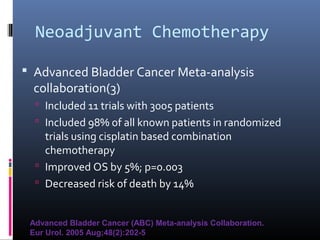
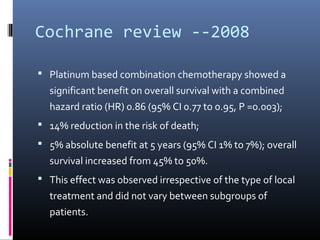
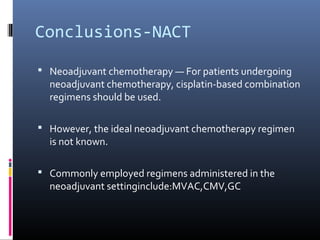
This document discusses chemotherapy options for metastatic bladder cancer. It notes that the prognosis remains poor with a median survival of 14 months. It reviews response rates of single agents like cisplatin, methotrexate, and doxorubicin. It then discusses combination regimens like MVAC (methotrexate, vinblastine, doxorubicin, and cisplatin), noting response rates of around 50% and median survival of approximately 12 months based on several studies. Larger phase 3 trials found MVAC improved median survival compared to cisplatin or cisplatin/cyclophosphamide regimens.






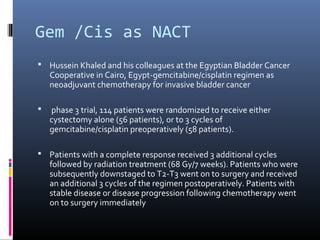
![ reported an overall response of 56% (30% complete
response [CR] and 25% partial response [PR]), and
bladder preservation was possible in 22% of those
patients who had a CR.
They also noted a trend toward increased 1-year survival
in the chemotherapy patients relative to cystectomy-
alone patients (69% vs 54%, P = .9)](https://image.slidesharecdn.com/chemotherapyincaub2-140710114856-phpapp02/85/Chemotherapy-in-ca-urinary-bladder-dr-prasanta-dash-16-320.jpg)





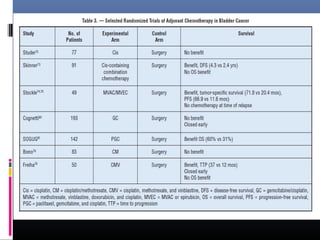
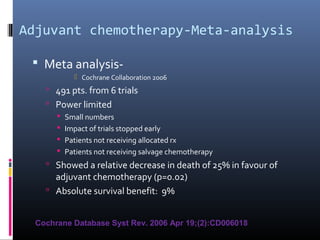
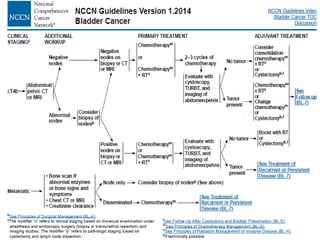
![Spanish Oncology GU Group
Trial 99/01
The Spanish Oncology Genitourinary Group trial 99/01 planned
to randomly assign 340 patients with T3-T4 or node positive
disease to treatment using four cycles of paclitaxel, gemcitabine,
and cisplatin (PGC) or observation .
However, the trial was terminated in 2007 because of poor
accrual after only 142 patients were enrolled.
The preliminary results of this trial were presented at the 2010
American Society of Clinical Oncology (ASCO) meeting. At a
median follow-up of 51 months, adjuvant PGC resulted in a
significant increase in overall survival (OS) at five years compared
to no chemotherapy (60 versus 30 percent, hazard ratio [HR]
0.44).
Paz-Ares LG, Randomized phase III trial comparing adjuvant PGC to observation in patients with
resected invasive bladder cancer: J Clin Oncol 2010; 28:18s](https://image.slidesharecdn.com/chemotherapyincaub2-140710114856-phpapp02/85/Chemotherapy-in-ca-urinary-bladder-dr-prasanta-dash-28-320.jpg)


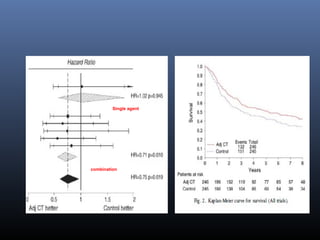
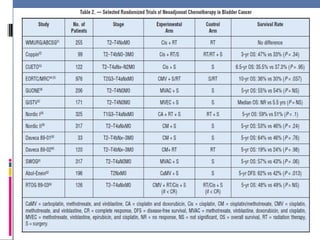
![Adj CT - EORTC Trial
EORTC Trial — A very different phase III study
which aimed to randomly assign 660 patients to
observation or treatment with adjuvant
chemotherapy (either methotrexate, vincristine,
doxorubicin, cyclophosphamide [M-VAC] or High
Dose M-VAC [HD-MVAC] or GC at the discretion
of the treating physician).
After enrollment of 248 patients, the trial was
closed due to slow accrual (though this surpasses
the accrual of patients in other contemporary
trials).](https://image.slidesharecdn.com/chemotherapyincaub2-140710114856-phpapp02/85/Chemotherapy-in-ca-urinary-bladder-dr-prasanta-dash-31-320.jpg)