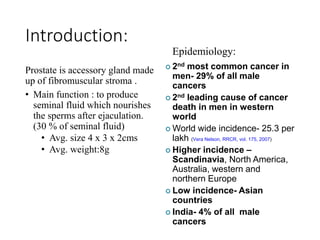
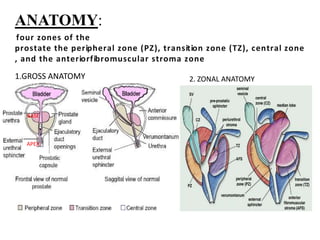
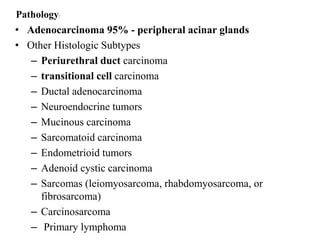
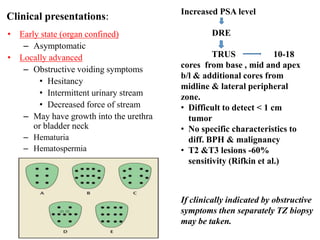
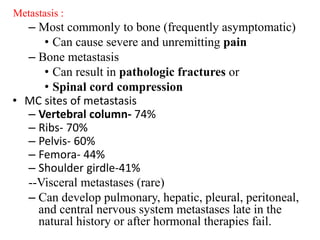
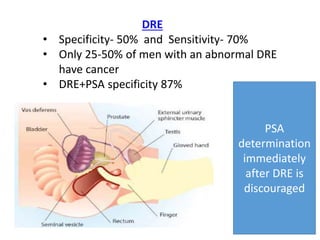
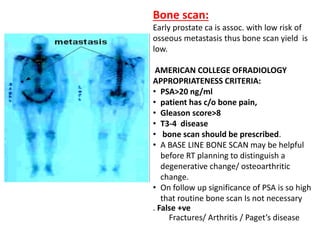
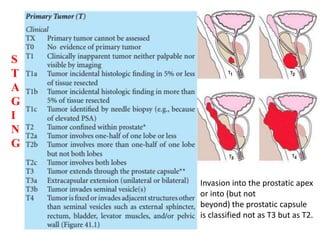
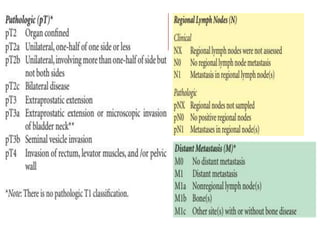
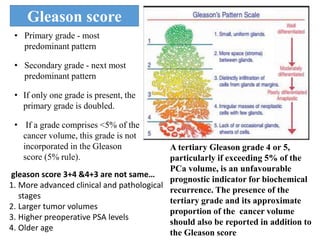
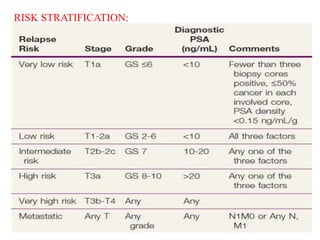
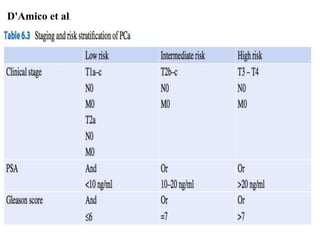
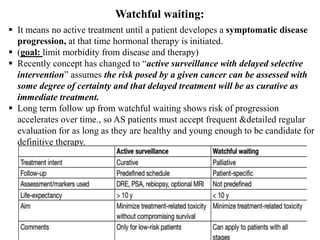
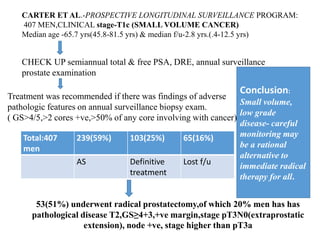
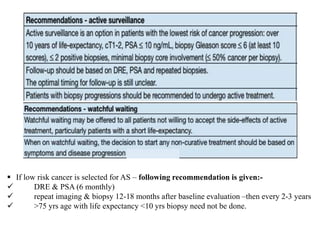
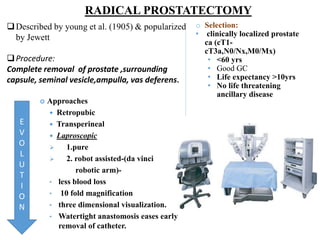
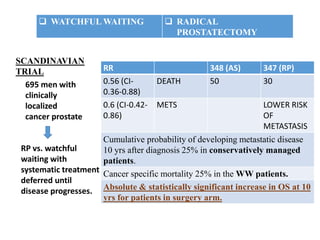
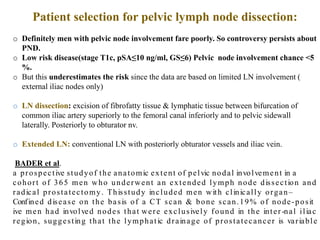
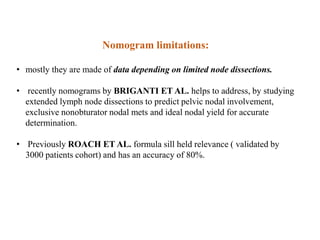
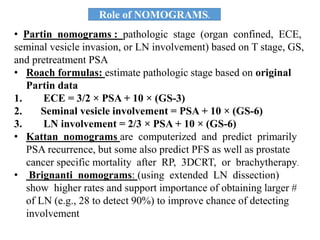
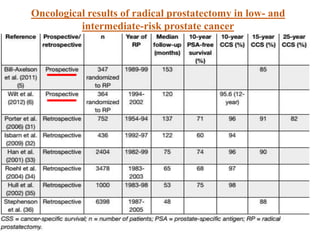
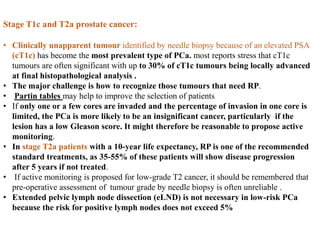
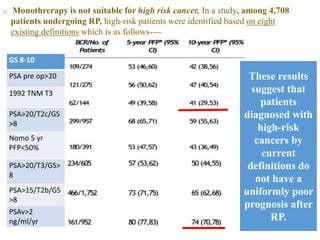
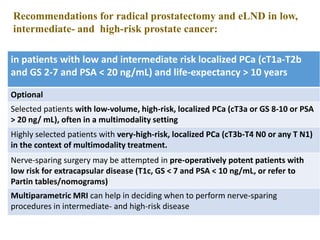
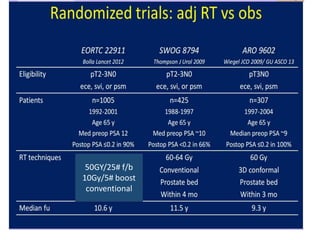
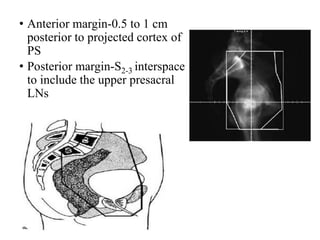
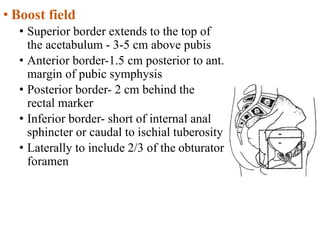
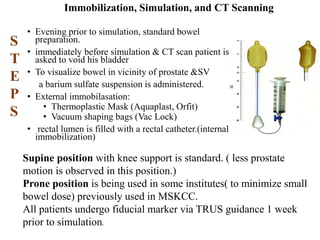
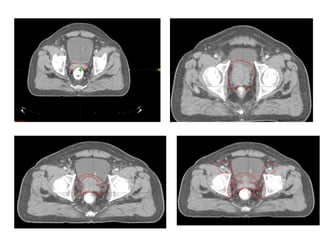
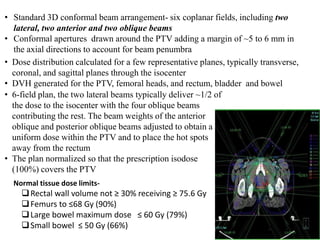
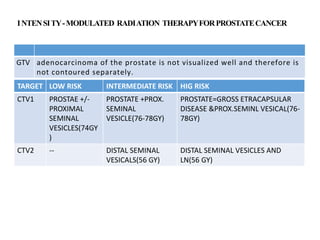
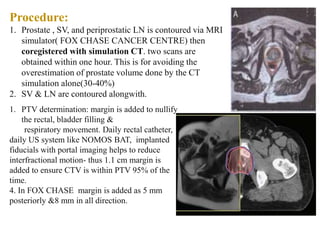
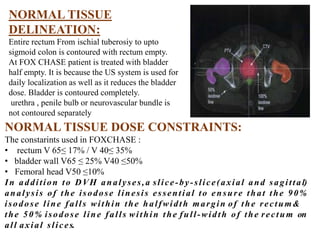
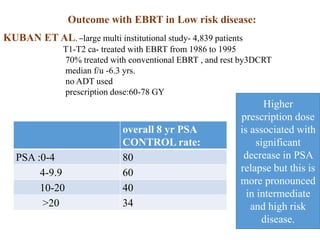
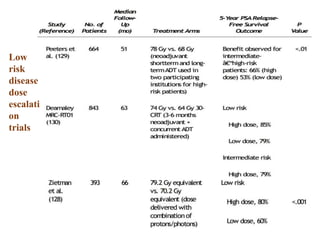
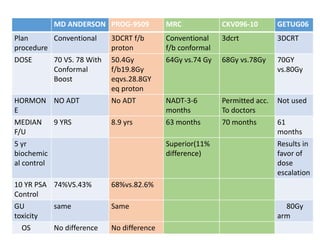
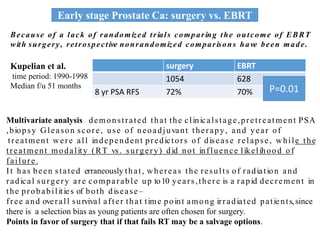
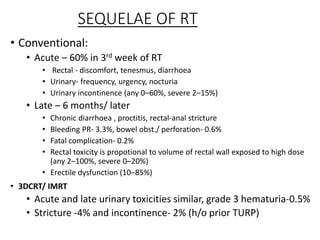
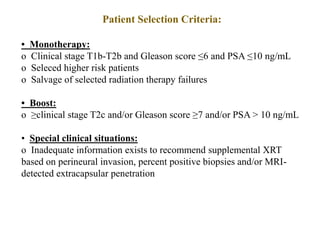
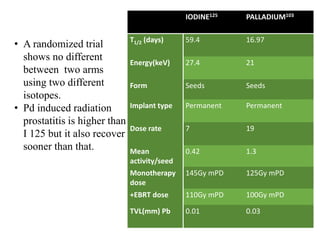
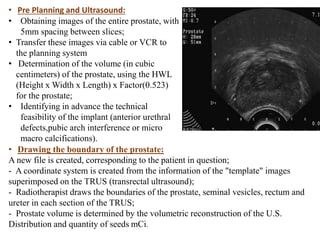
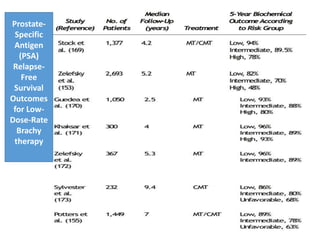
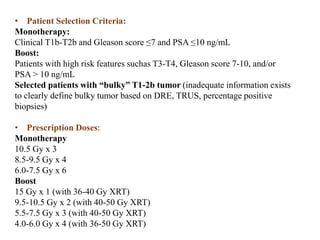
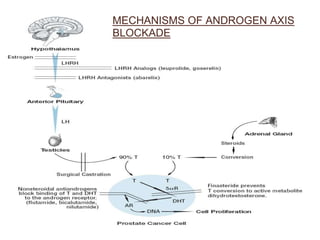
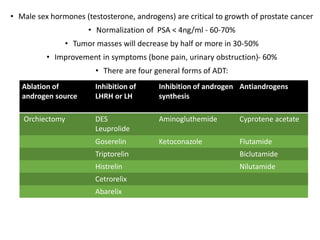
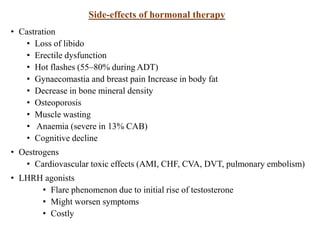
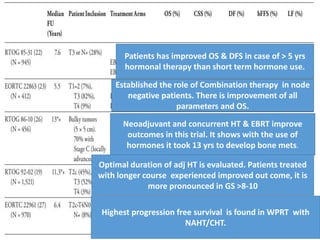
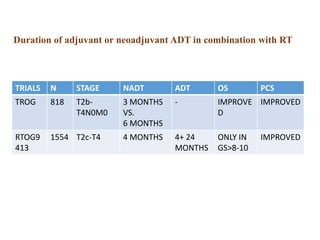
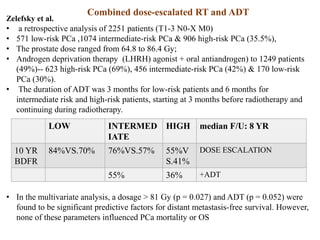
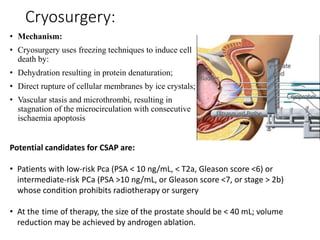
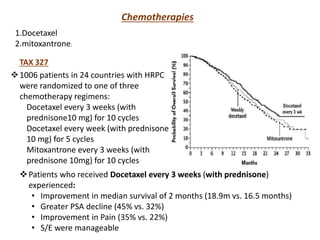
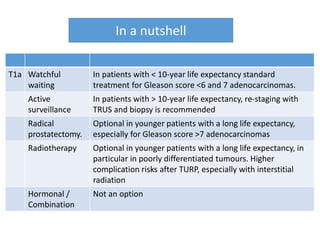
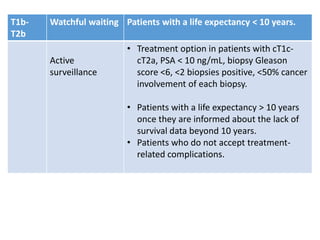
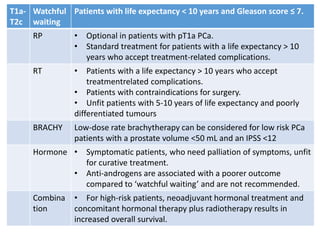
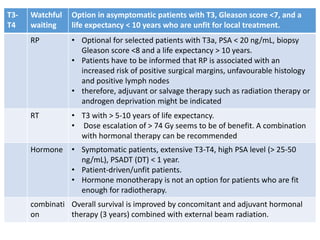
The prostate is a gland that produces seminal fluid. Prostate cancer is the second most common cancer in men. The prostate has four zones - peripheral, transition, central and anterior fibromuscular. Prostate cancer usually arises in the peripheral zone and is typically an adenocarcinoma. Diagnosis involves a digital rectal exam, prostate-specific antigen testing, transrectal ultrasound of the prostate and biopsy. Staging involves evaluating if the cancer is organ-confined or has spread locally or metastasized. Treatment options depend on risk stratification and may include active surveillance, surgery, radiation therapy or hormone therapy.