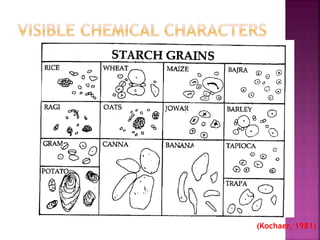
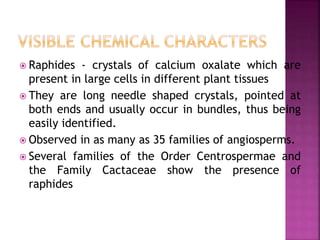
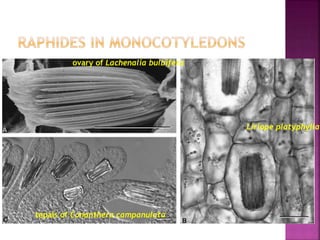
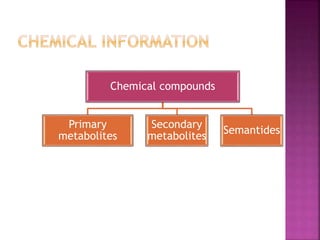
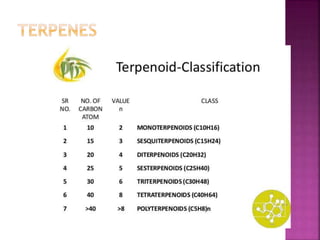
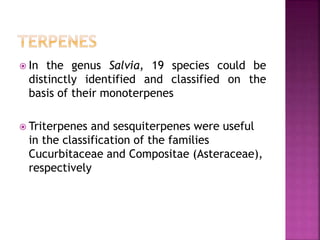
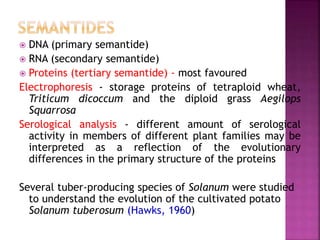
This document discusses various chemical compounds that can be used for chemotaxonomic analysis of plants, including primary metabolites, secondary metabolites, and semantides. Primary metabolites like carbohydrates and amino acids are universally found but not very useful taxonomically. Secondary metabolites like phenolics, alkaloids, and terpenoids are more restricted in occurrence and greater importance for taxonomy. Specific examples of using flavonoids, monoterpenes, triterpenes, sesquiterpenes, and proteins in classifying and identifying plant species are provided.