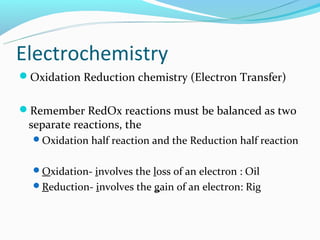
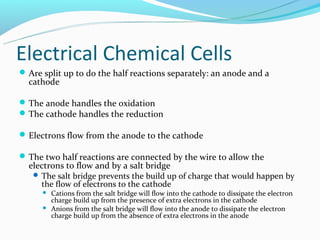
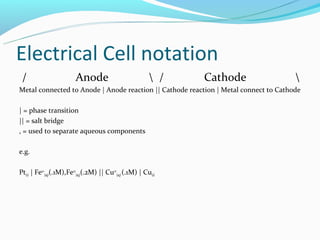
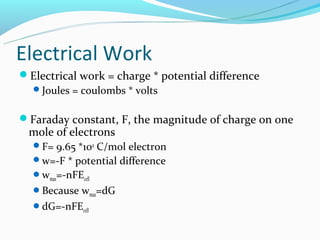
The document discusses electrochemistry concepts including oxidation-reduction reactions, electrical chemical cells, galvanic cells, standard cell notation, standard cells, and the Nerst equation. Key points include: redox reactions involve separate oxidation and reduction half reactions; cells split these half reactions between an anode and cathode connected by a salt bridge; cell potential is measured in volts and relates to Gibbs free energy; and the Nerst equation allows predicting non-standard cell potentials based on reaction quotient concentrations.