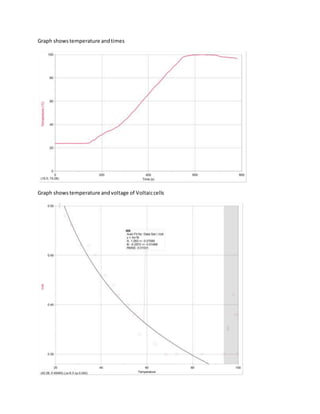
1. The document describes an experiment to investigate how temperature impacts the flow of electrons and current in a voltaic cell.
2. The voltaic cell consisted of zinc and iron half-cells connected by a salt bridge, and the independent variable was temperature. The hypothesis was that higher temperatures would increase the reaction rate and voltage.
3. The results showed that voltage actually decreased with increasing temperature above 90 degrees, possibly due to measurement errors at very high temperatures, though lower temperatures generated more voltage overall.