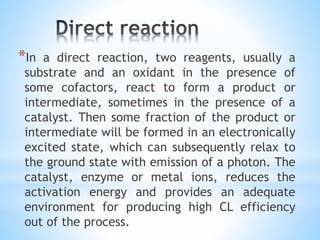
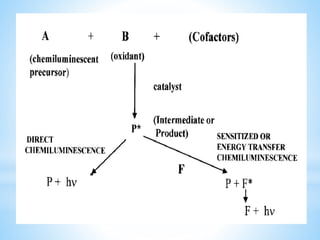
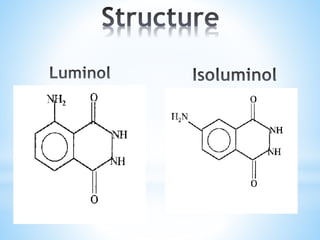
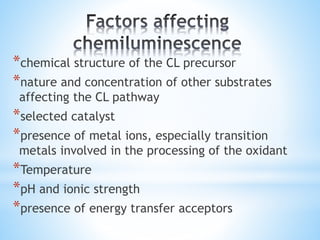
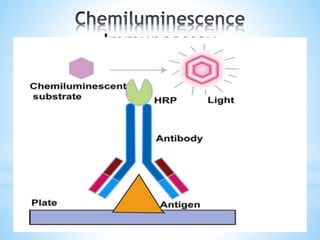
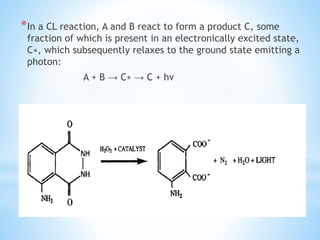
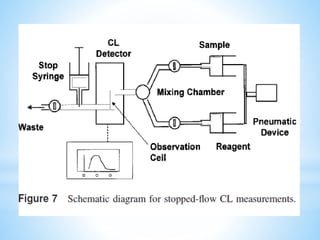
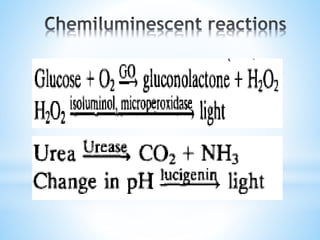
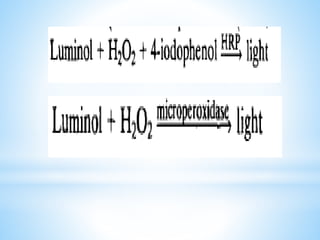
Chemiluminescence is the emission of light from a chemical reaction and can be used for analytical testing. It involves reactions that produce electronically excited molecules that then decay to ground state while emitting photons of light. Common substances used include luminol, acridinium esters, and ruthenium derivatives. When oxidized, typically with hydrogen peroxide, intermediates are produced in excited states. As they return to ground state, light is emitted. This light can be measured with high sensitivity for applications like immunoassays, liquid chromatography detection, and clinical diagnostics. Advantages include stability, low toxicity, high sensitivity down to attomole and zeptomole levels, and fast results.



![* It is the light emission that is produced in
certain chemical oxidation reactions.
[A] + [B] → [◊] → [Products] + light](https://image.slidesharecdn.com/immunoassay-150715092846-lva1-app6891/85/Immunoassay-4-320.jpg)