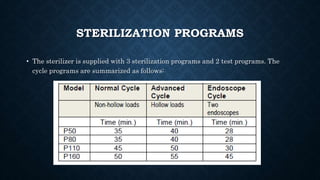
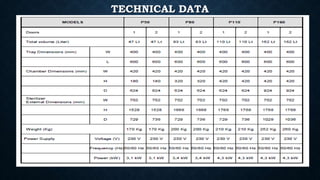
This document describes a plasma sterilizer. It begins by explaining what sterilization and plasma sterilization are. Plasma sterilization uses mechanisms like free radicals, UV/VUV radiation, and volatilization to eliminate microorganisms. It then discusses different methods of plasma sterilization like dielectric barrier discharge, inductively coupled plasma, and atmospheric pressure plasma jets. The document concludes by providing details about a specific low-temperature plasma sterilizer model made by Tuttnaurer, including its features, sterilization programs, and use of a process challenge device.