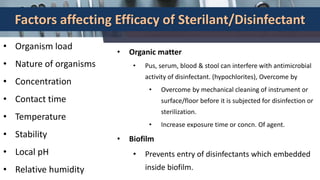
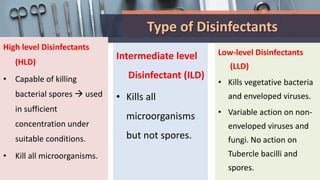
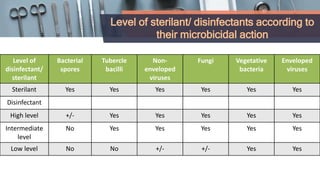
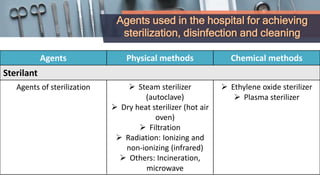
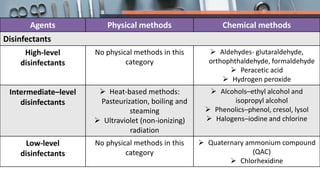
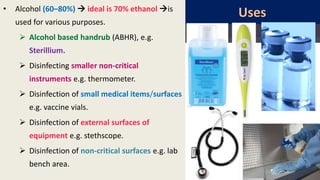
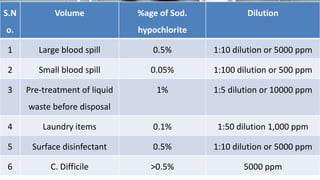
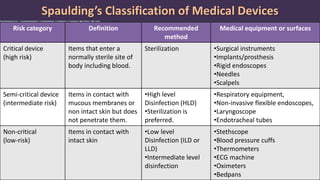
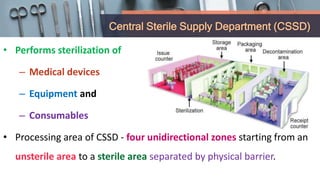
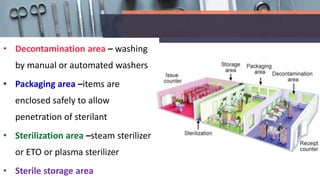
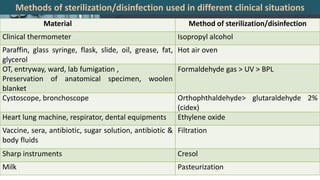
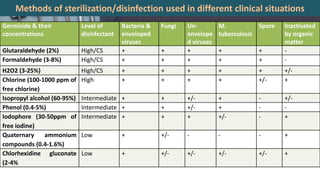
This document defines various terms related to disinfection and sterilization such as sterilization, disinfection, antiseptics, sanitizers, asepsis, and provides classifications of different disinfection methods and agents used for achieving sterilization and disinfection in hospitals. It also discusses factors affecting the efficacy of sterilants/disinfectants and properties of an ideal disinfectant.