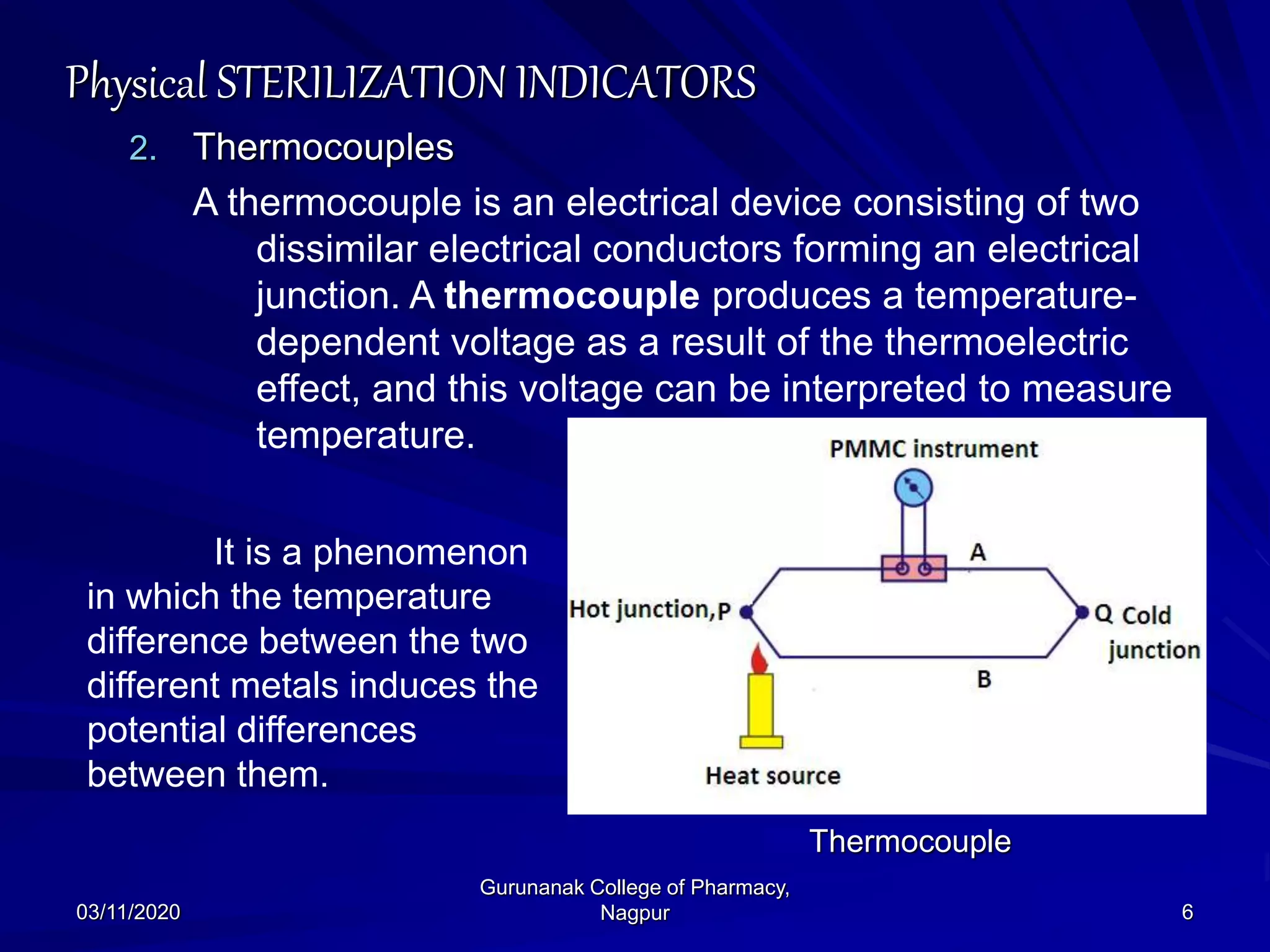
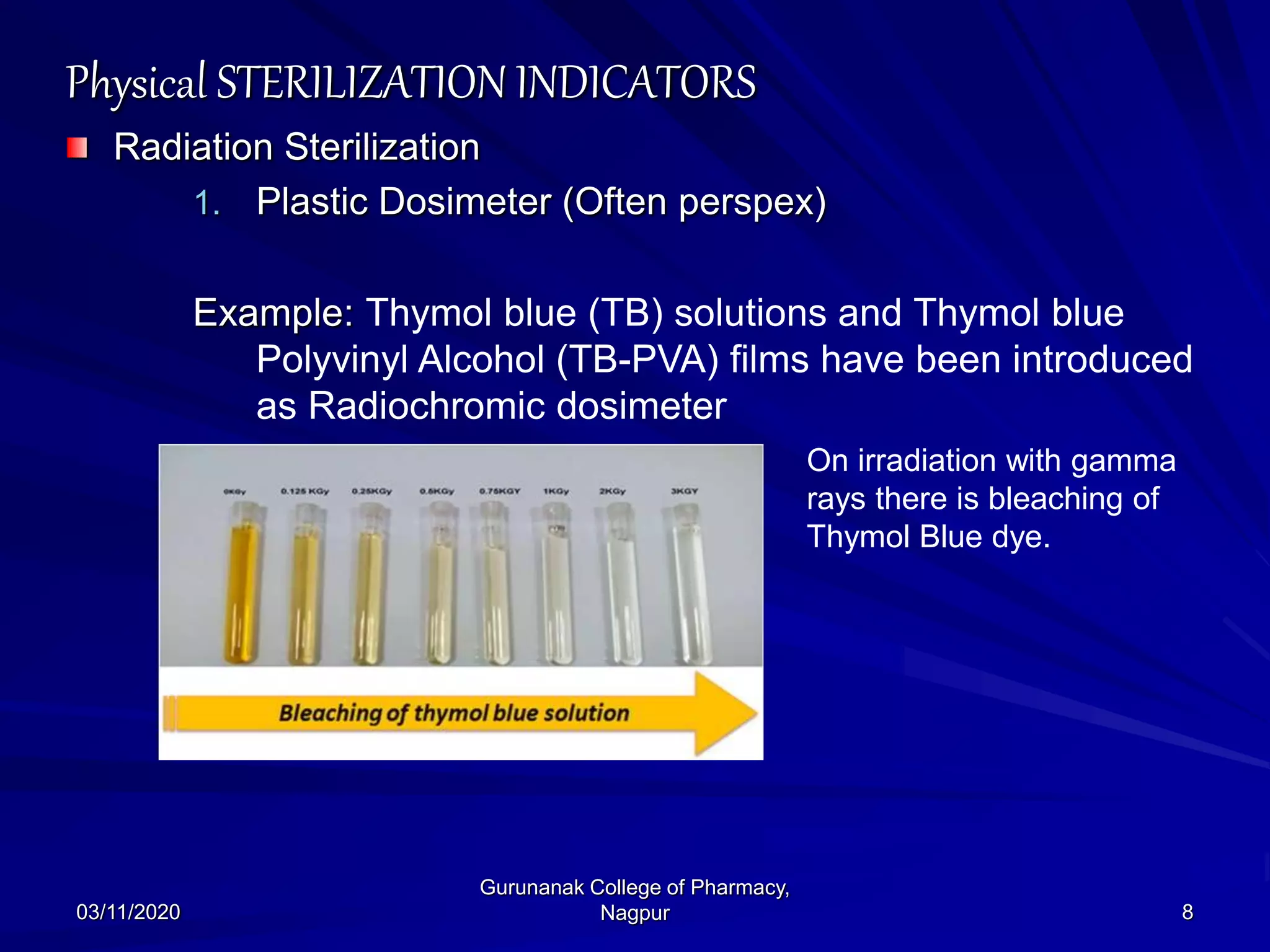
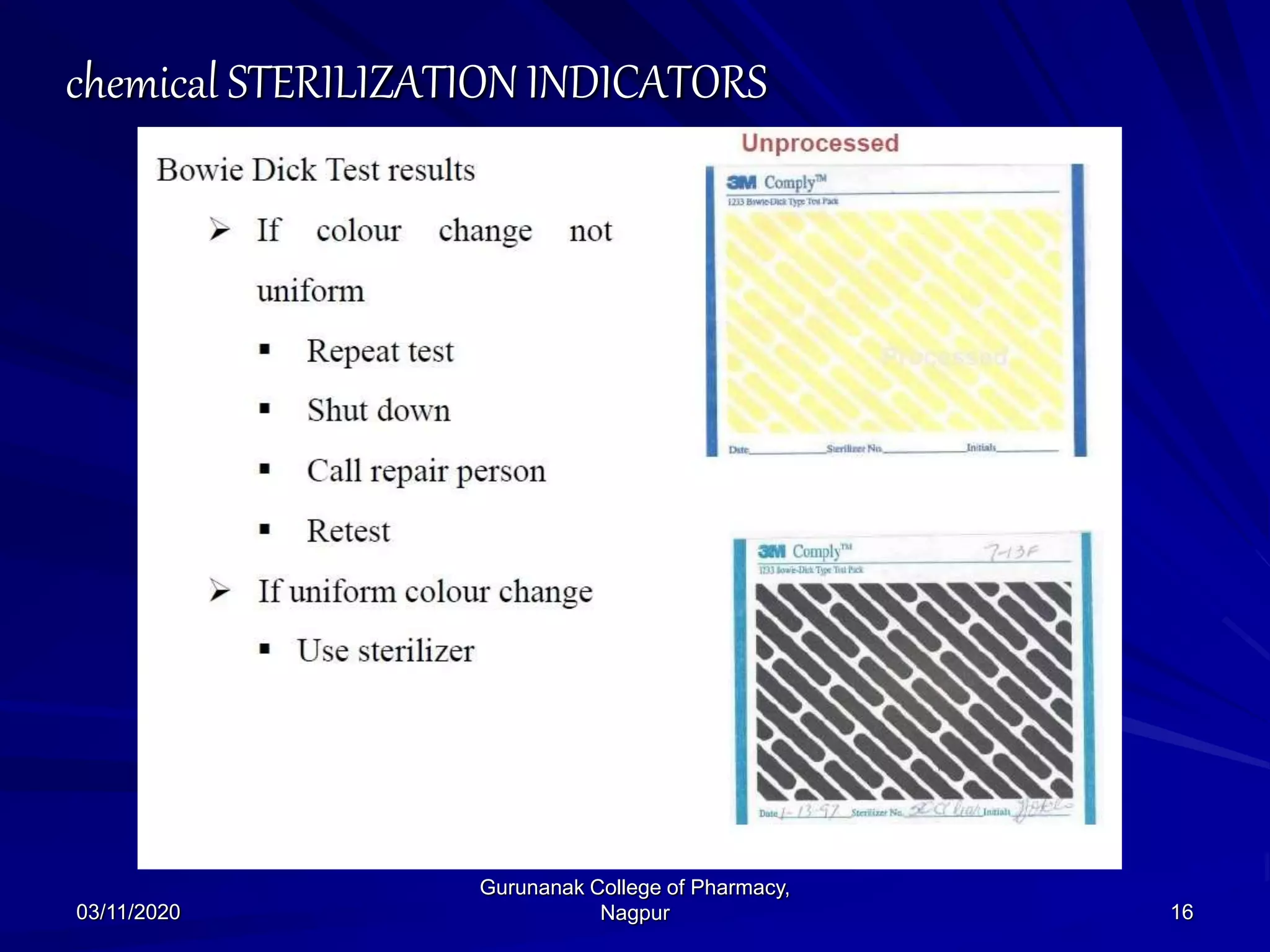
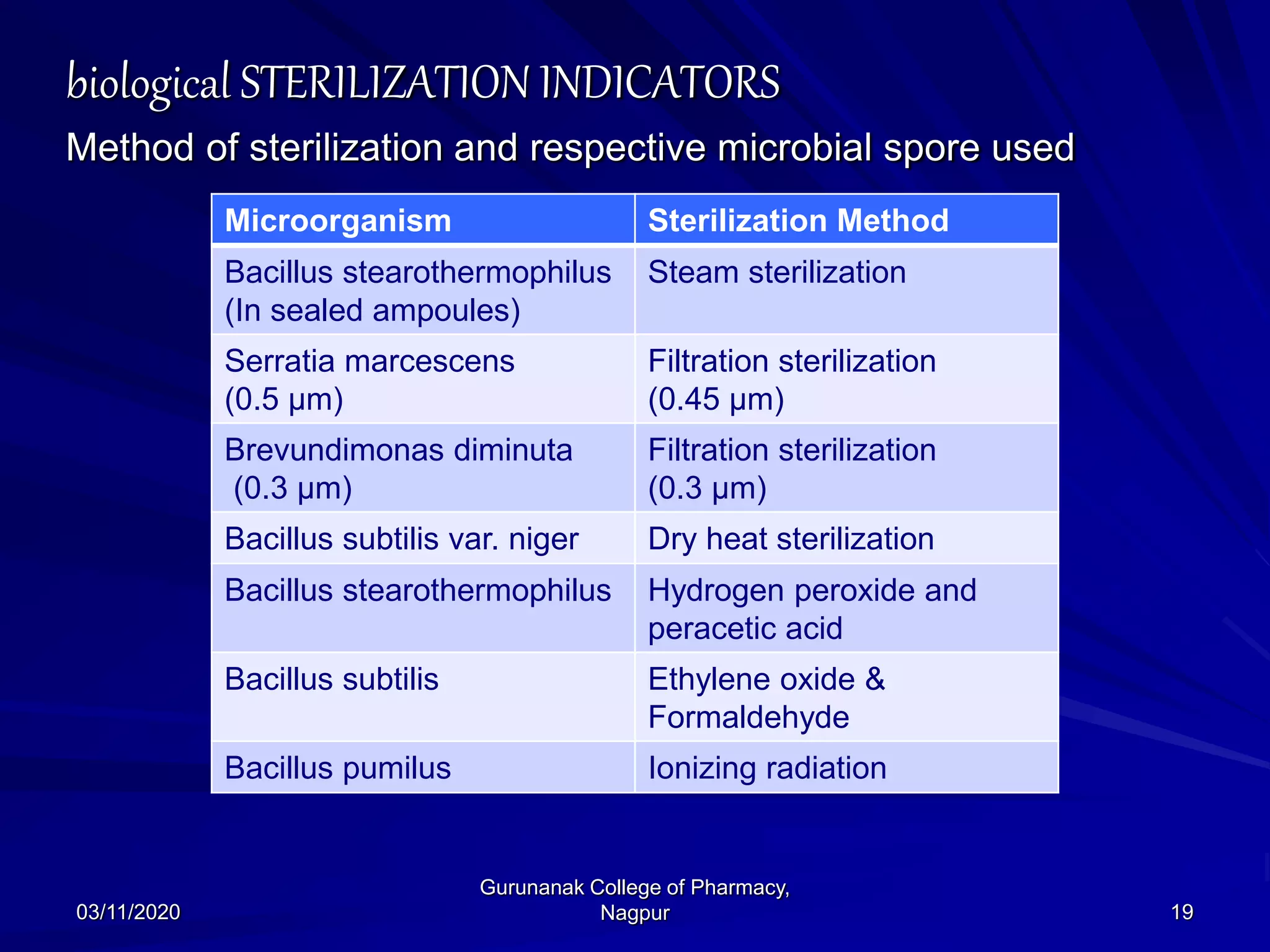
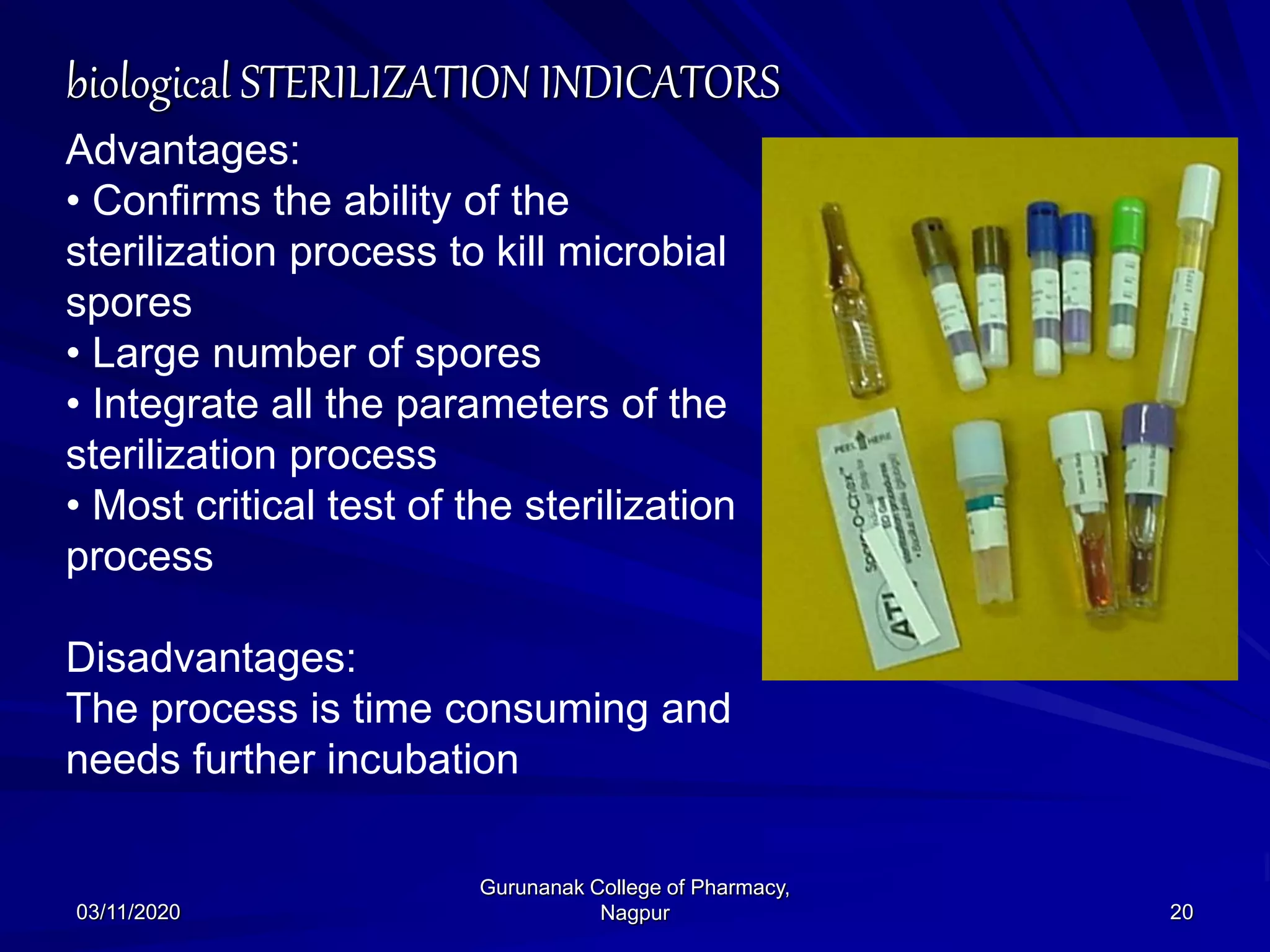
The document discusses sterilization indicators used to confirm the effectiveness of sterilization processes, categorizing them into physical, chemical, and biological types. Physical indicators rely on parameters like temperature and pressure measurements, while chemical indicators change properties during sterilization, and biological indicators use bacterial spores to validate sterility. Each type has specific methods and advantages, highlighting the importance of ensuring proper sterilization conditions.