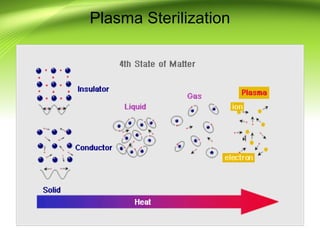
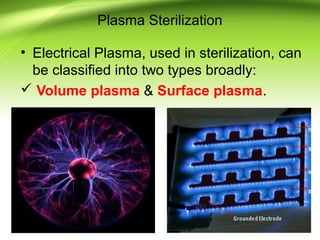
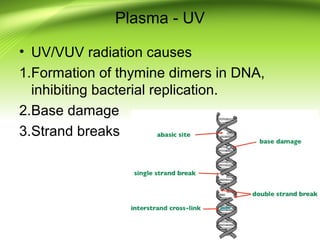
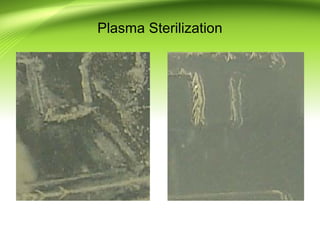
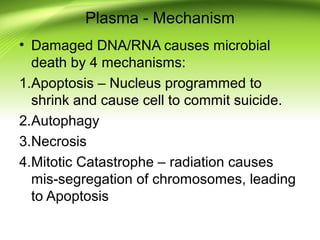
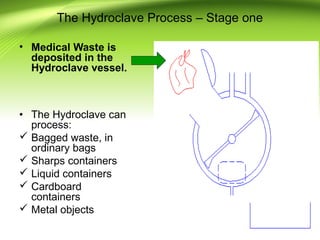
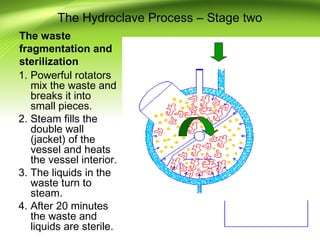
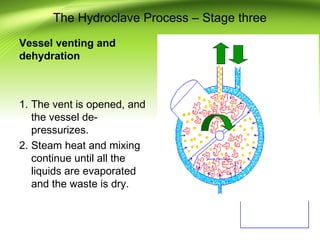
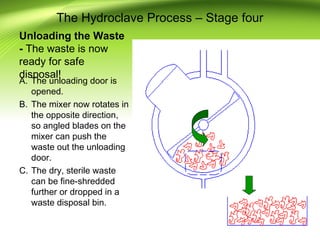
The document discusses recent advances in sterilization methods, focusing on plasma and gas plasma sterilization. Plasma sterilization uses ionized gas or plasma to kill microorganisms rapidly through UV photons, ions, electrons and chemical reactions. It has advantages over conventional methods like steam sterilization in being faster, safer and more versatile. However, challenges include weak penetration and material compatibility issues. Gas plasma systems like STERRAD use vaporized hydrogen peroxide and plasma to sterilize in under an hour without residues. Pulsed light also uses brief high intensity UV flashes to destroy microbes. Research continues to improve understanding and applications of non-thermal sterilization methods.