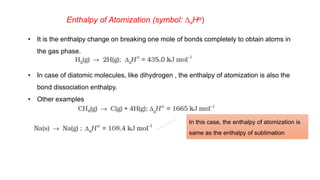
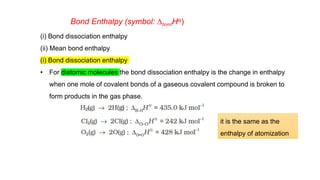
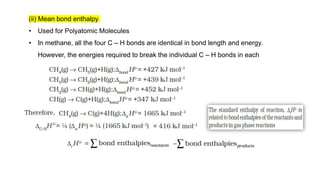
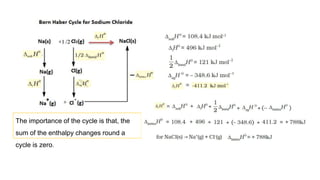
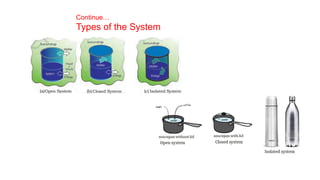
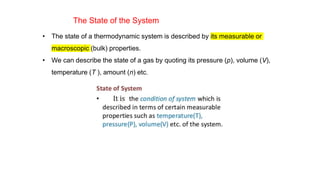
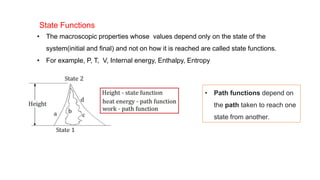
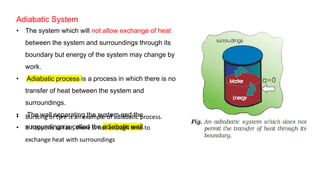
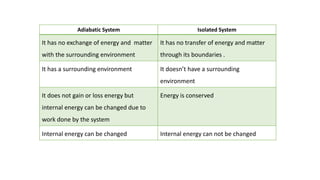
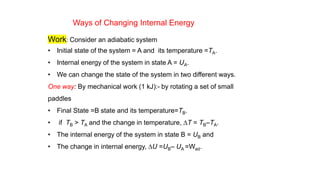
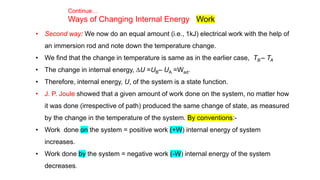
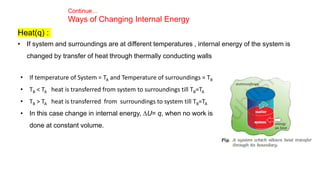
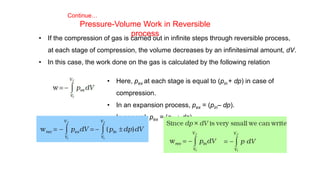
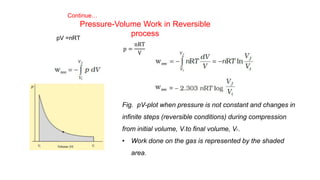
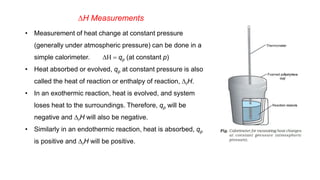
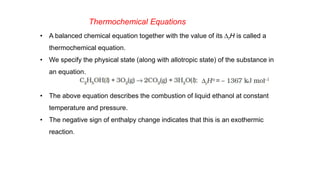
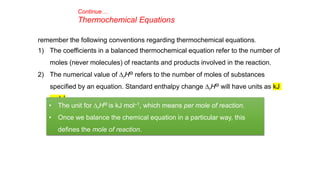
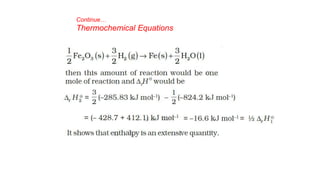
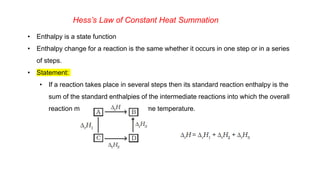
This document covers thermodynamics, focusing on key concepts such as the system and surroundings, types of thermodynamic systems (open, closed, isolated), and state functions. It explains internal energy, the first law of thermodynamics, pressure-volume work, and processes like reversible and irreversible processes. Additionally, it discusses enthalpy and its relationship to internal energy, including mathematical formulations and examples related to changes in states of systems.







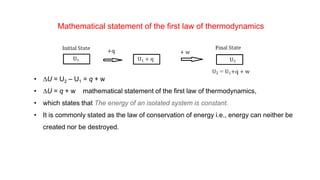



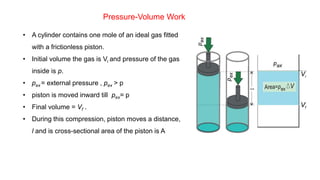
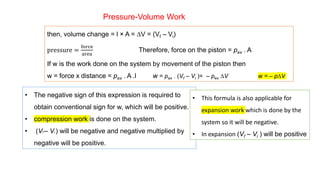


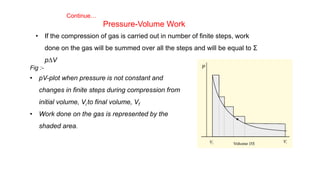





![Enthalpy, H
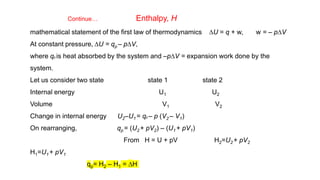
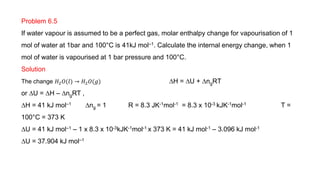
• The heat absorbed at constant volume is equal to change in the Internal Energy i.e.,
DU = qv
• But most of chemical reactions are carried out not at constant volume, but in flasks or
test tubes under constant atmospheric pressure.
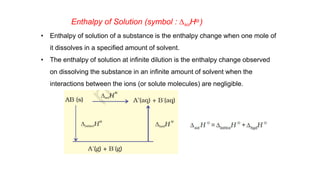
• Another thermodynamic function, the enthalpy H [Greek word enthalpien, to warm or
heat content] as : H = U + pV
• Enthalpy is the sum of internal energy and product of pressure-volume.
• Enthalpy is a state function-depends on initial and final states of the system
• Absolute value of H can not be determined.
• Change in enthalpy (ΔH) is determined. ΔH = H2-H1](https://image.slidesharecdn.com/class11chapter6thermodynamics-230102105503-77b13671/85/Class-11-Chapter-6-Thermodynamics-pptx-34-320.jpg)














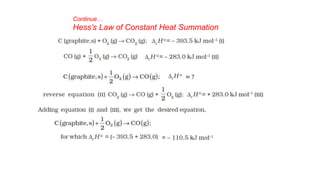

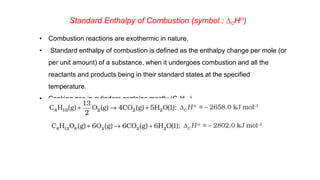

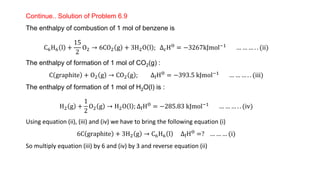

![Continue.. Solution of Problem 6.9
Alternate method
Given DfHƟ CO2(g) = –393.5 kJ mol–1 and DfHƟ H2O(l) = – 285.83 kJ mol–1 , DfHƟ
O2 g = 0
∆rH
Ɵ
= 6 × ∆fH
Ɵ
CO2 + 3 × ∆fH
Ɵ
H2O − [∆fH
Ɵ
C6H6 +
15
2
× ∆f H
Ɵ
O2 ]
−3267 = 6 × −393.5 + 3 × −285.83 − ∆fH
Ɵ
C6H6 + 0
−3267 = −2361 + −857.49 − ∆fH
Ɵ
C6H6 + 0
∆fH
Ɵ
C6H6 = −3218.49 + 3267 = 48.51k Jmol−1](https://image.slidesharecdn.com/class11chapter6thermodynamics-230102105503-77b13671/85/Class-11-Chapter-6-Thermodynamics-pptx-68-320.jpg)