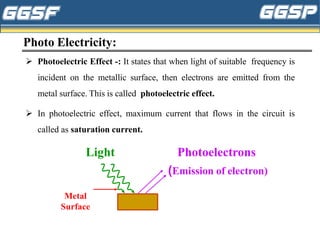
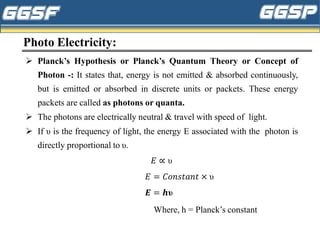
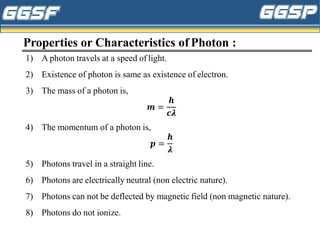
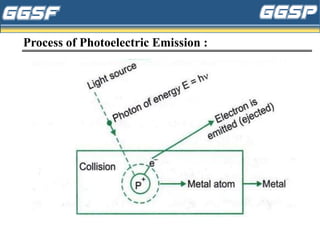
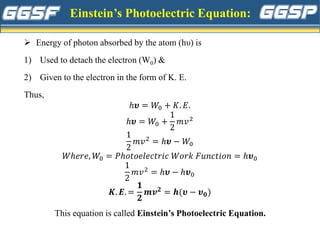
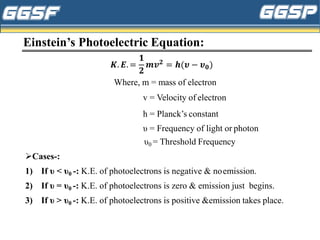
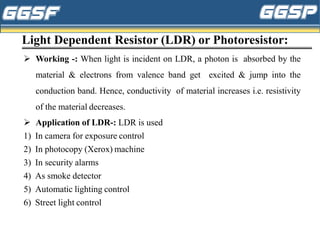
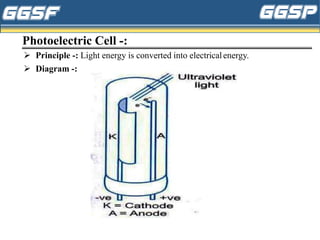
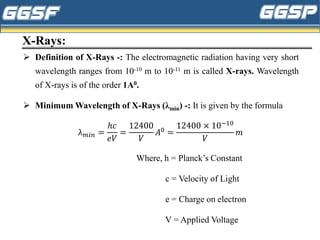
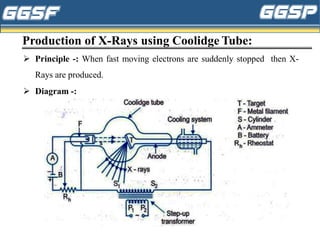
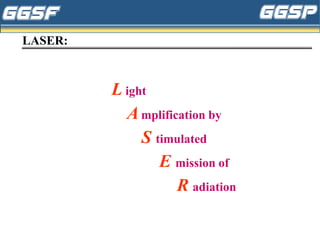
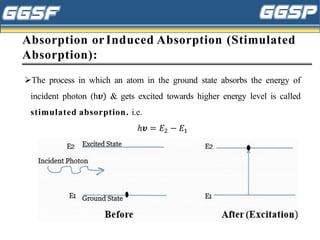
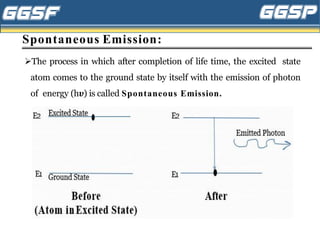
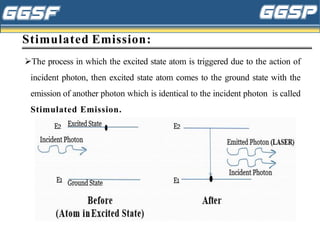
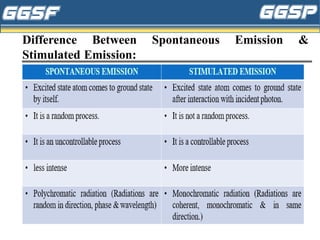
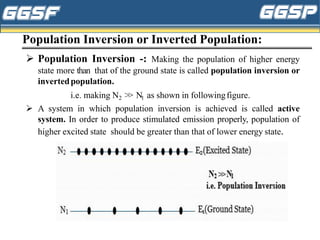
1) The document discusses topics related to photoelectricity, X-rays, and lasers including the photoelectric effect, Planck's quantum theory, Einstein's photoelectric equation, production of X-rays using a Coolidge tube, and characteristics of laser light.
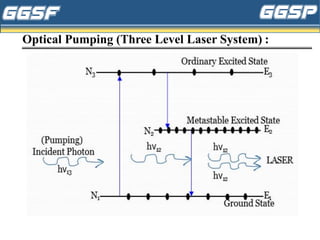
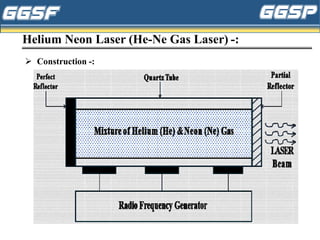
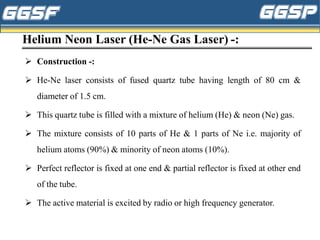
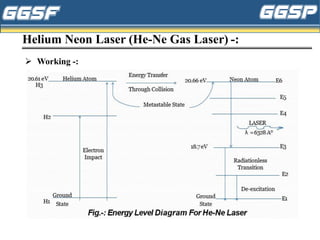
2) It describes the three-level optical pumping system used in helium-neon lasers, which produces population inversion through excitation from the ground state to a higher excited state followed by relaxation into a metastable state.
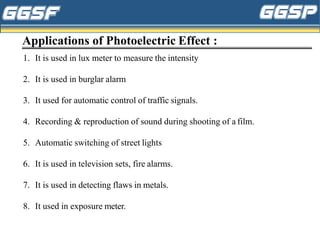
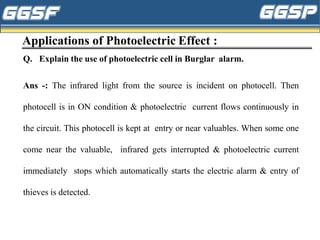
3) Applications of these phenomena are discussed, such as uses of photoelectric cells in burglar alarms, medical and industrial applications of X-rays, and examples of laser applications in areas like engraving, cutting,