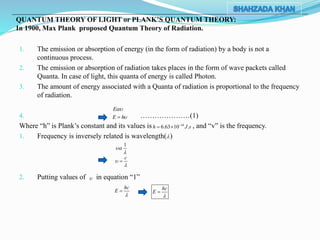
1) The document discusses the photoelectric effect and early explanations provided by Planck's quantum theory and Einstein. It describes experiments showing that electrons are emitted from metals when light above a threshold frequency strikes them.
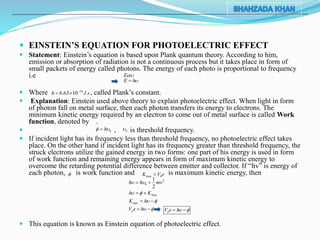
2) Einstein used Planck's idea that energy is emitted and absorbed in discrete quanta to explain the photoelectric effect. He proposed that light consists of discrete packets of energy called photons, and that photons impart their entire energy to electrons.
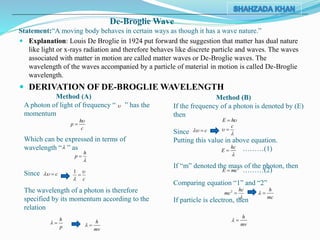
3) The document also discusses de Broglie's hypothesis that all matter exhibits wave-particle duality, and derives an expression for the de Broglie wavelength of matter particles.