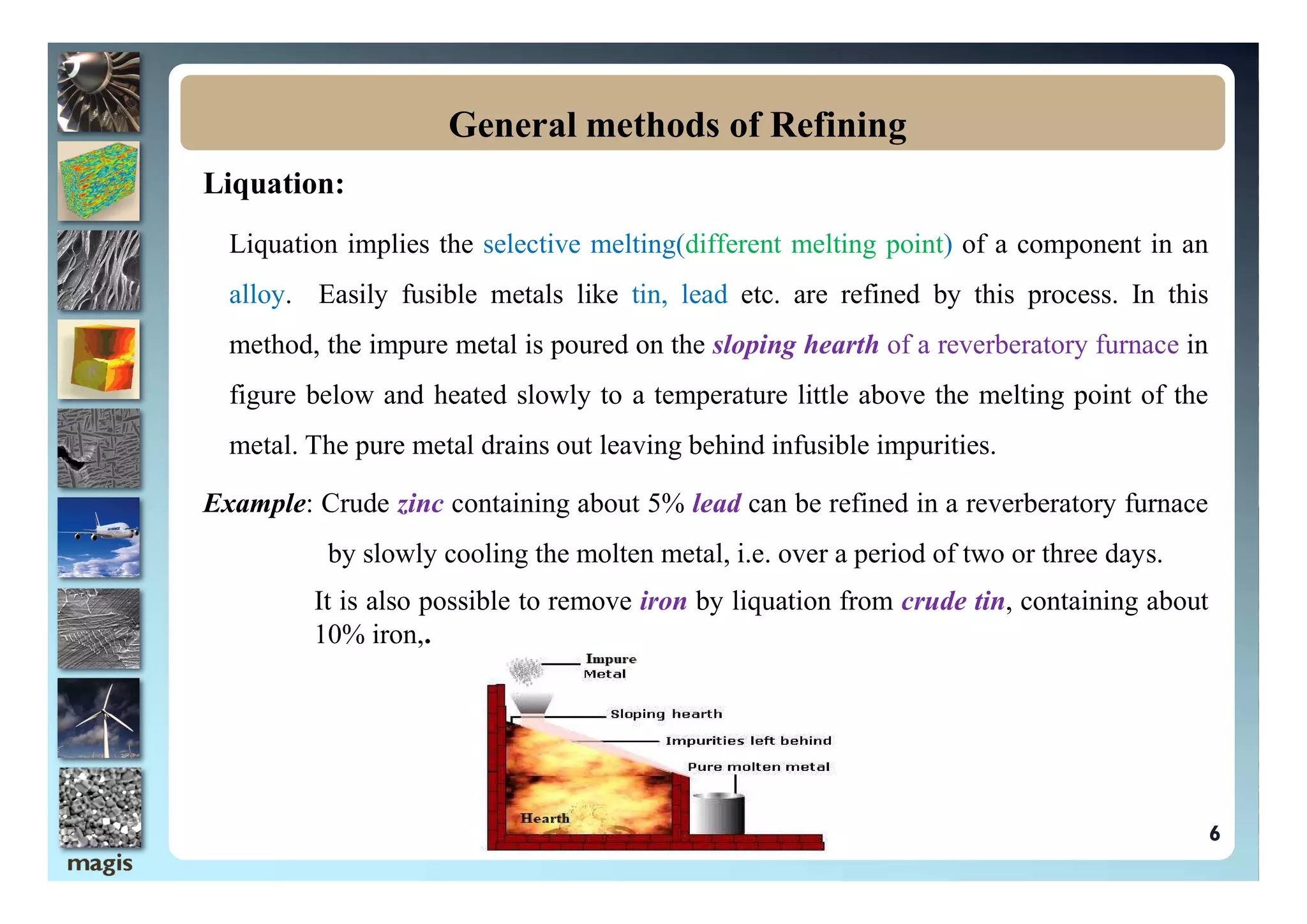
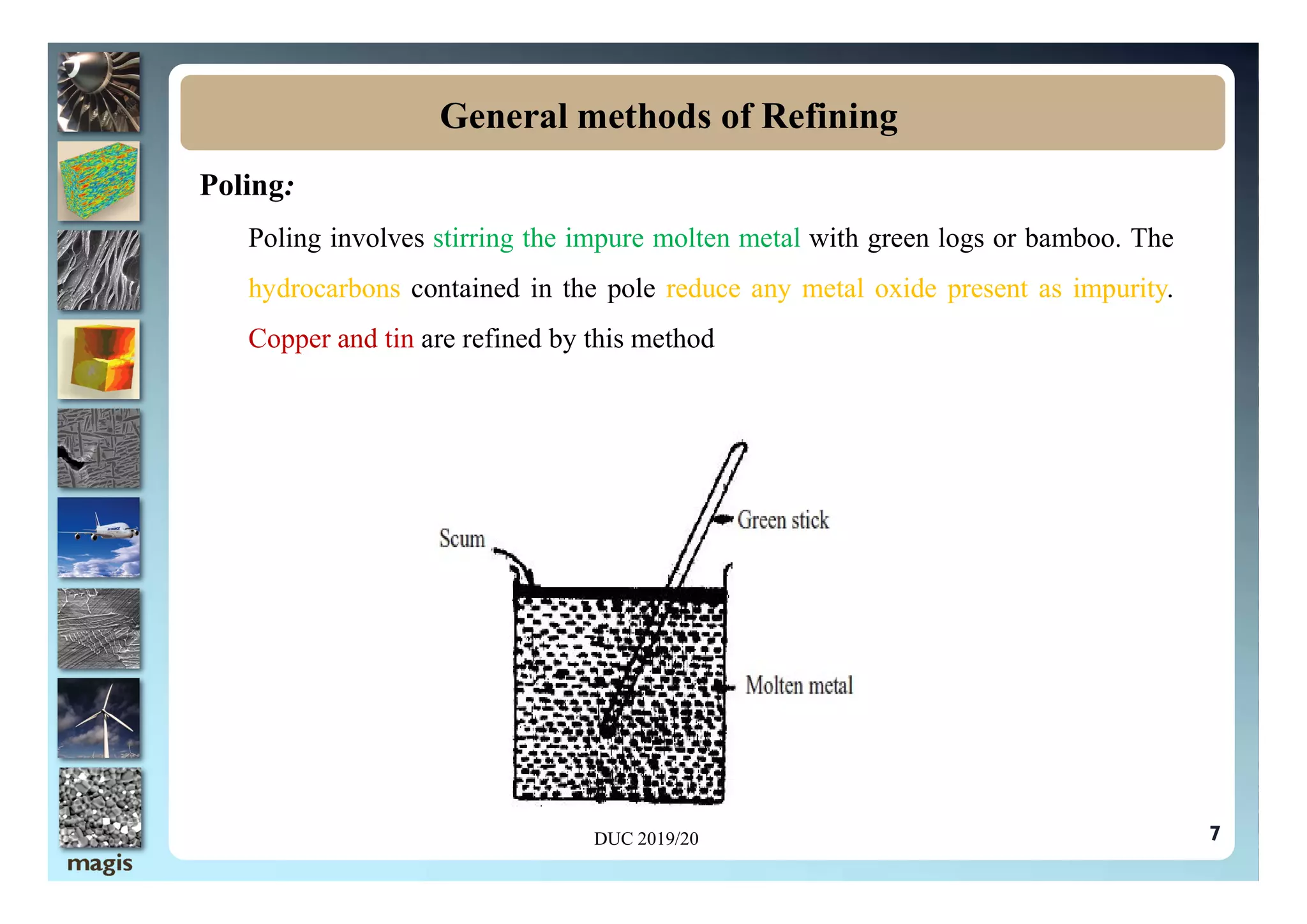
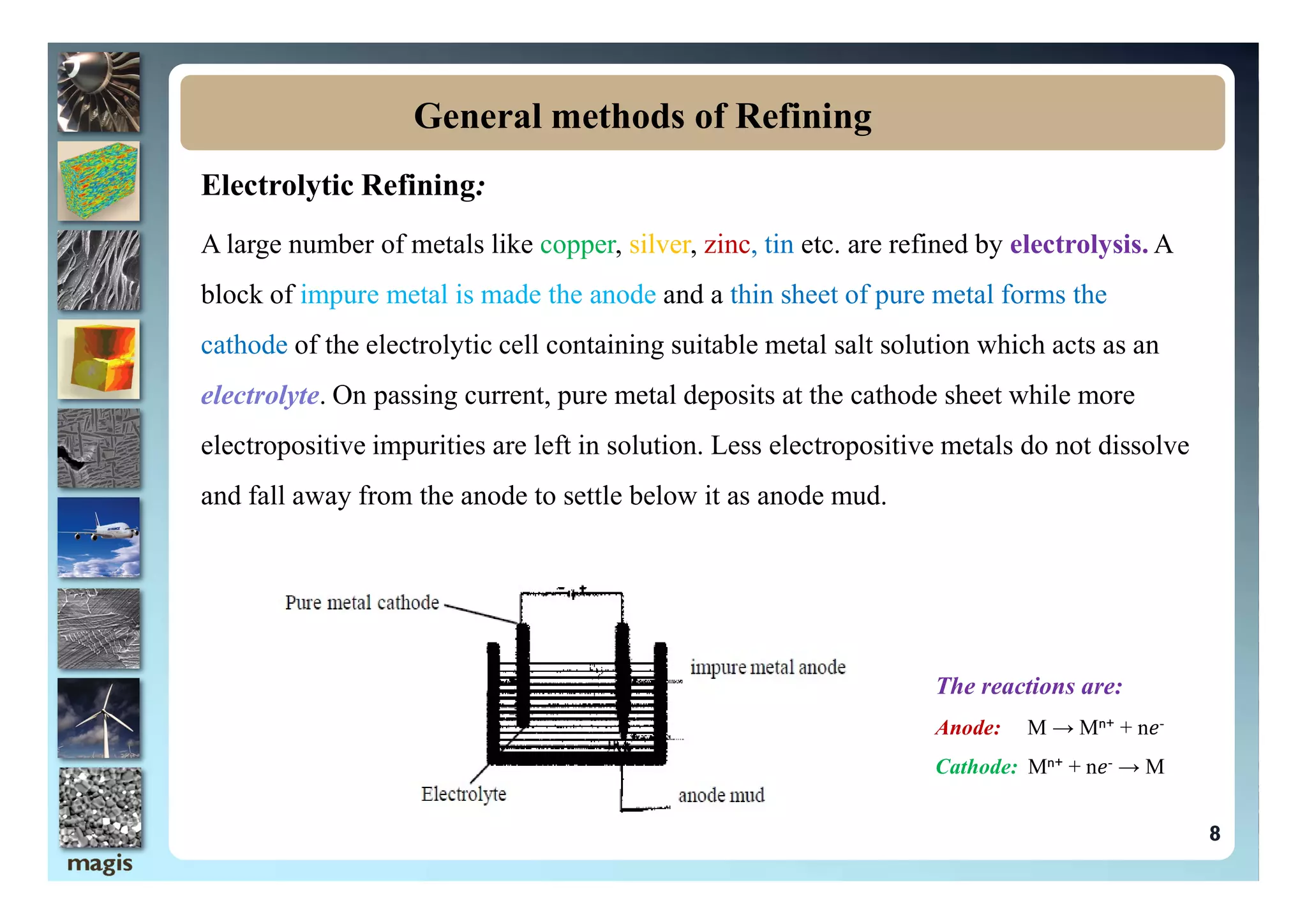
The document discusses the processes of refining and purifying metals, highlighting the importance of removing impurities to enhance desired properties such as mechanical strength and electrical conductivity. Various methods of refining, including distillation, liquation, electrolysis, and chromatographic techniques, are described alongside examples that illustrate their application. The document emphasizes that different metals may require specific refining methods based on their unique characteristics and the nature of the impurities present.