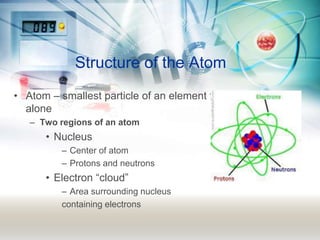
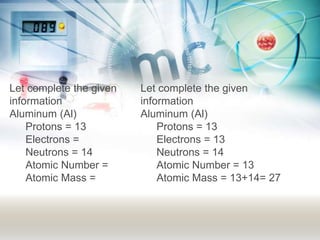
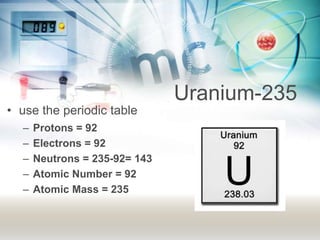
This document discusses the structure of atoms. It explains that atoms are made up of a nucleus containing protons and neutrons, surrounded by an electron cloud containing electrons. The atomic number is the number of protons, which determines the element. The mass number is the total number of protons and neutrons. Examples are provided to demonstrate how to determine the atomic number and mass of different elements using their nuclear symbols or the periodic table.