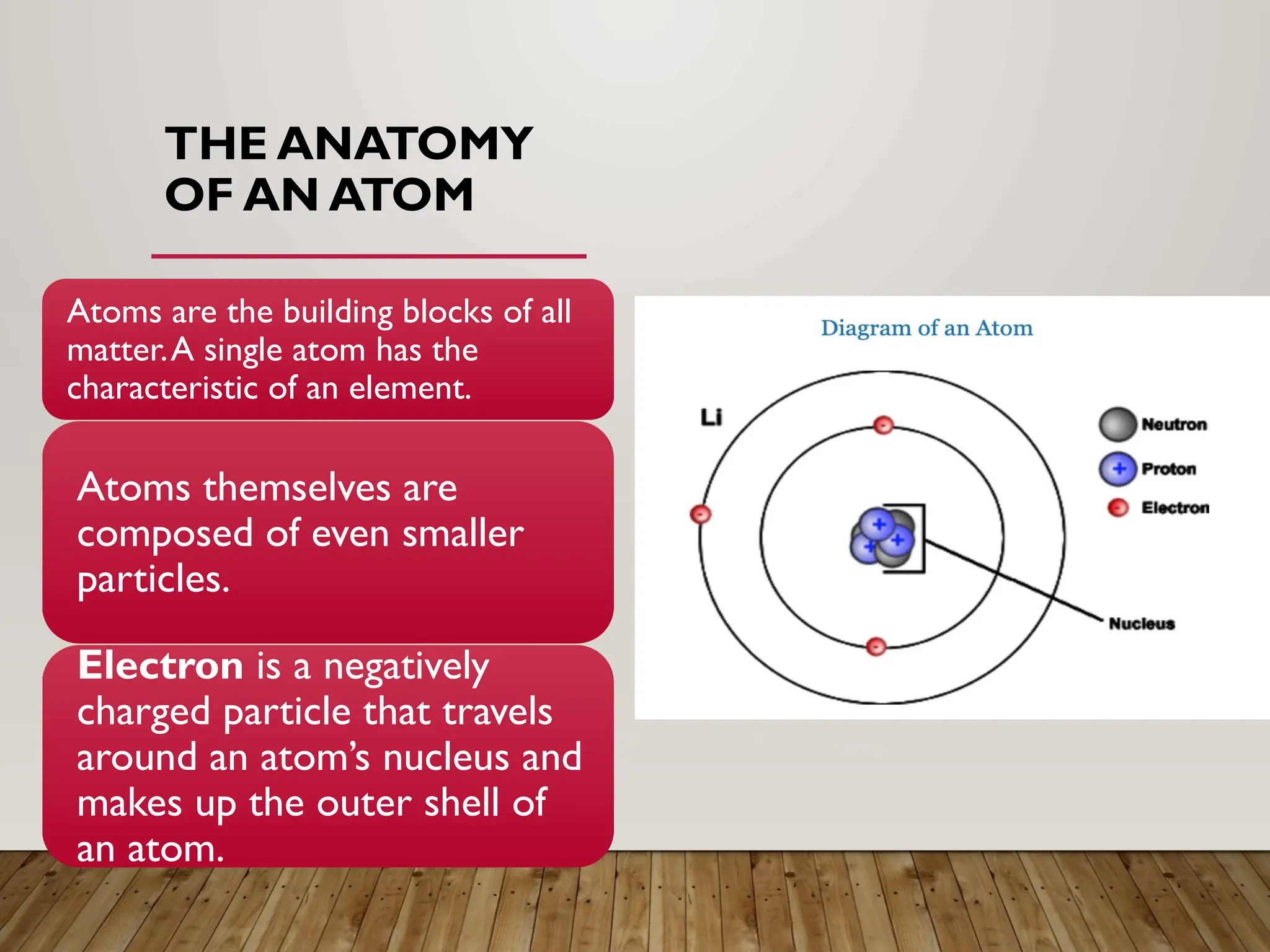
The document explains the structure of atoms, detailing subatomic particles such as electrons, protons, and neutrons, and how they contribute to elements on the periodic table. It discusses concepts like isotopes, atomic number, and mass, as well as electronegativity and the unique properties of noble gases. The periodic table serves as a tool to understand the relationships and behaviors of different elements based on their atomic structure.