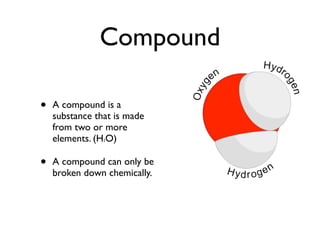
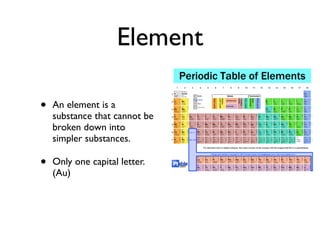
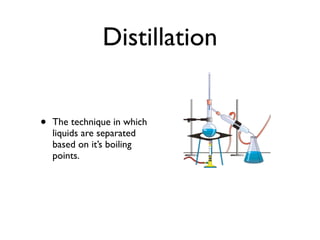
The document defines key chemistry concepts such as elements, compounds, mixtures, solutions, suspensions, colloids, and separation techniques including distillation, evaporation, and filtration. It also discusses chemical changes and provides examples such as changes in color, temperature, production of gas, or formation of precipitate. Physical and chemical changes are compared. Reactivity and flammability are also addressed. Review questions are included to test understanding.