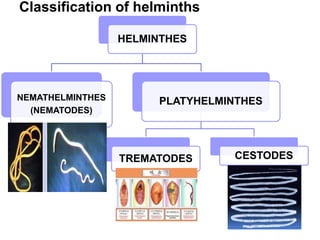
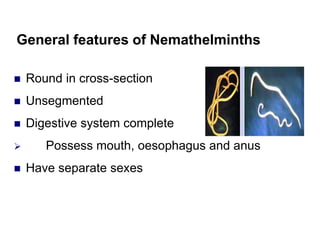
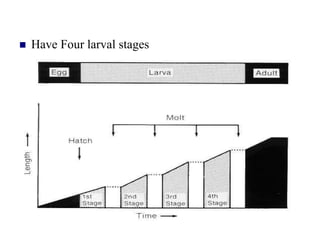
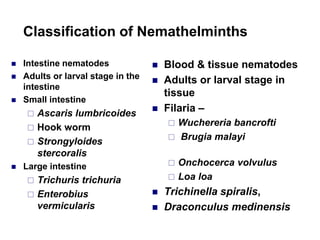
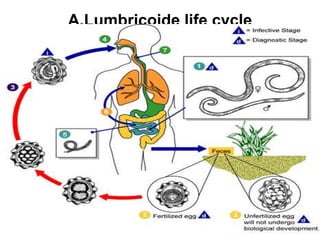
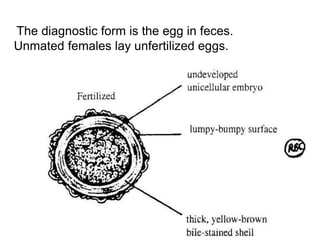
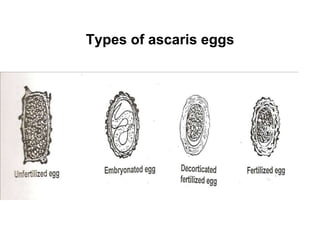
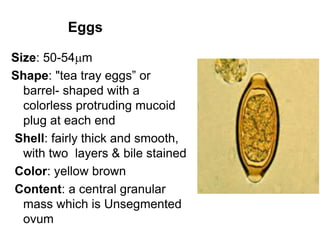
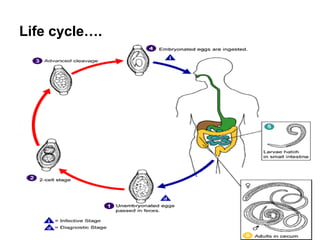
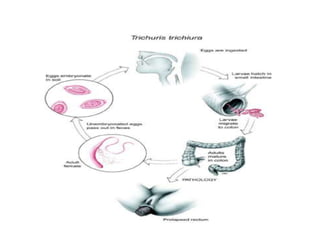
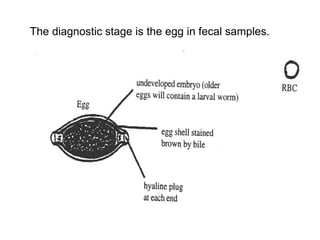
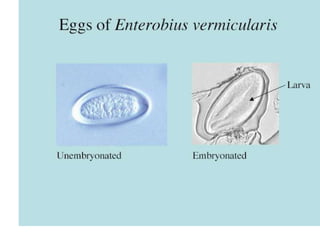
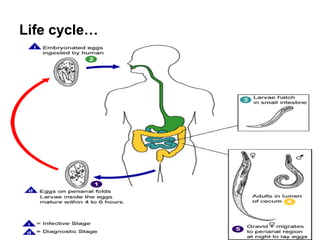
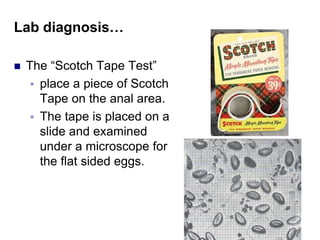
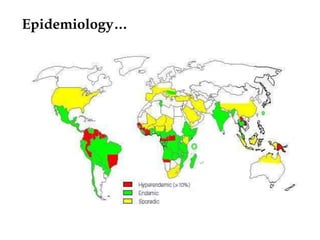
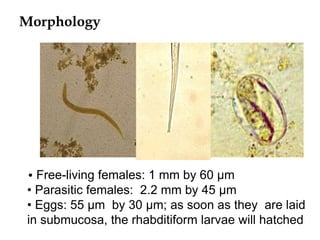
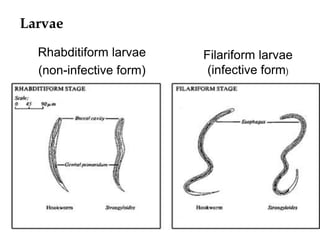
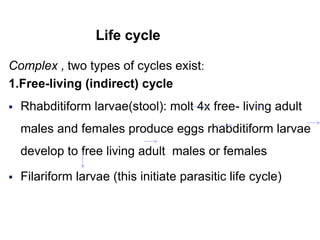
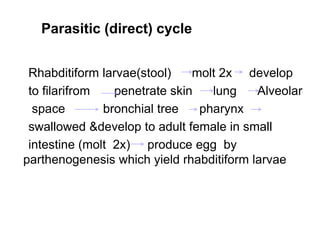
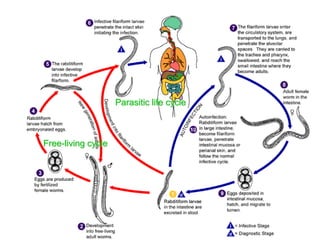
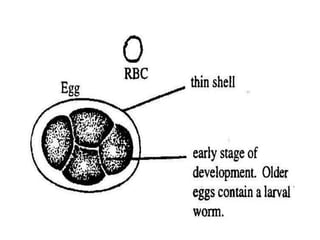
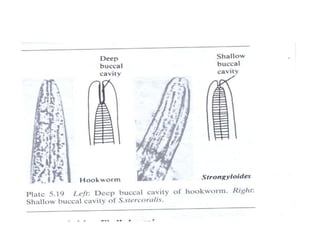
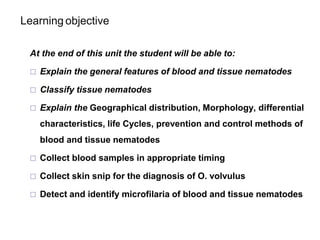
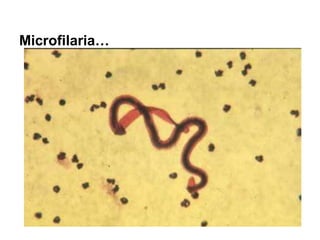
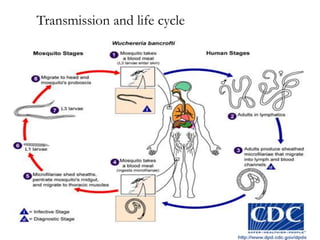
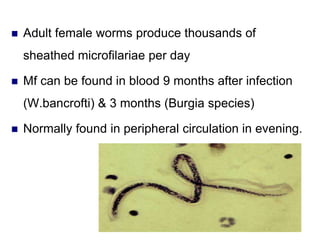
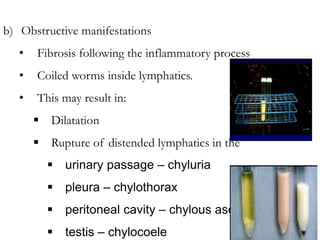
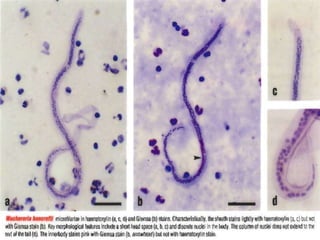
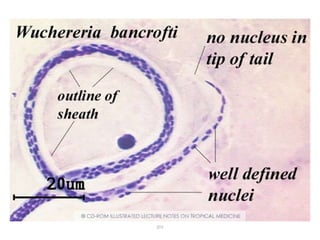
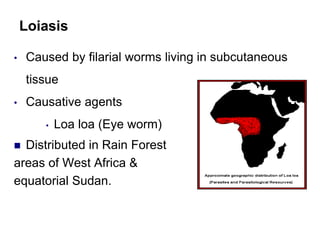
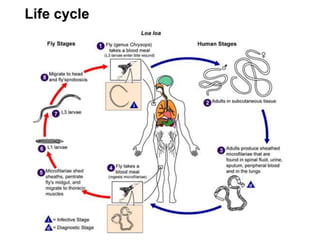
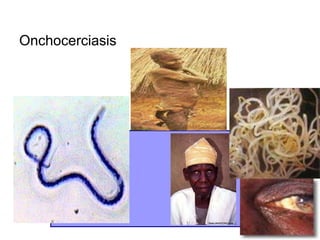
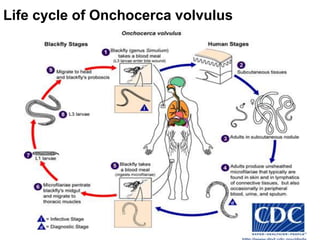
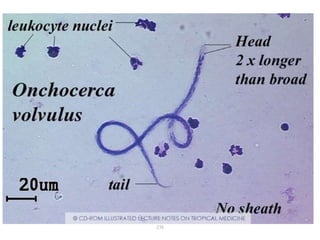
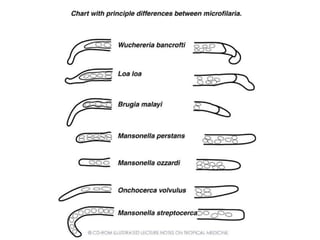
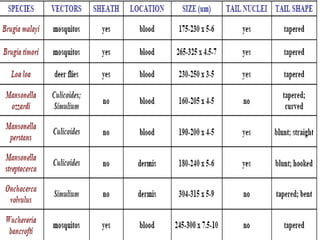
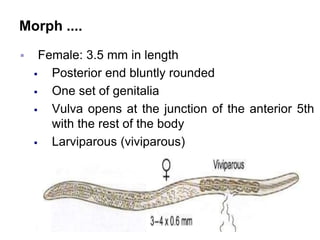
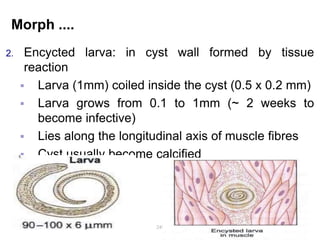
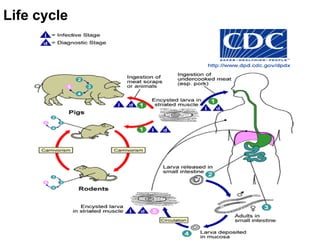
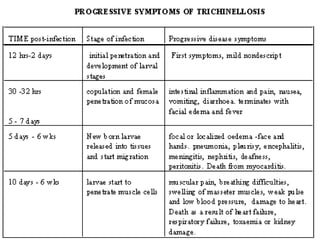
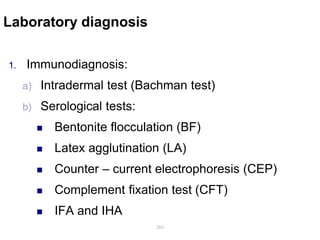
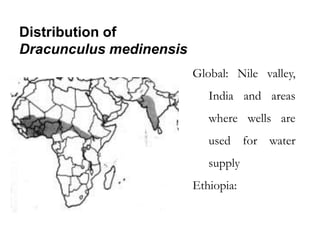
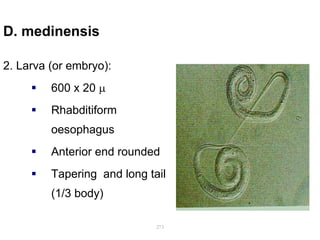
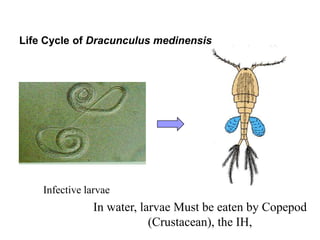
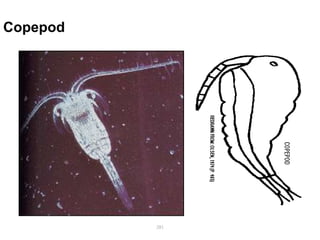
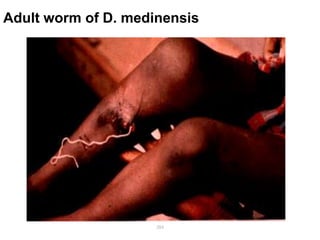
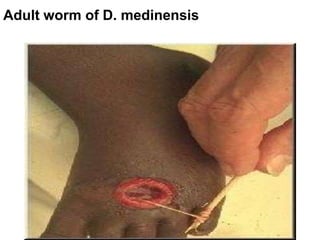
The document provides information about helminths (parasitic worms). It begins by defining helminths and outlining the learning objectives and classification of helminths. The document then focuses on intestinal nematodes (roundworms), describing their key features, life cycles, transmission routes, and important examples like Ascaris lumbricoides and Trichuris trichiura. It discusses the morphology, epidemiology, pathogenesis, laboratory diagnosis and treatment of these common helminth infections.