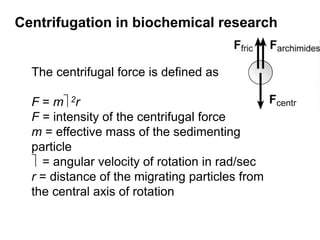
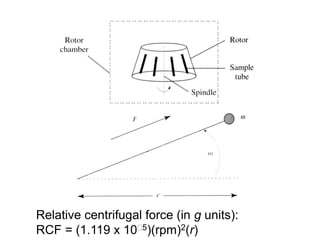
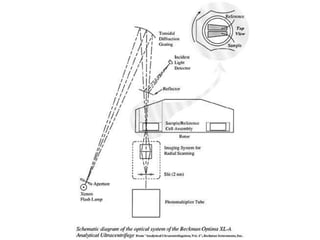
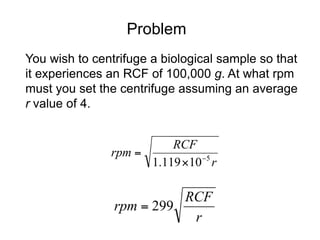
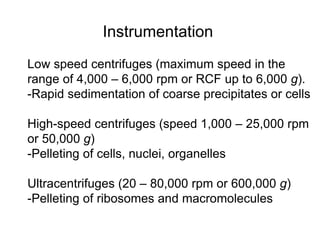
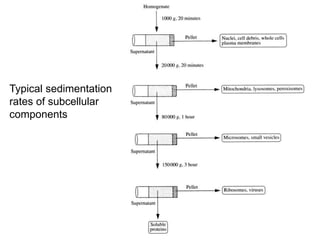
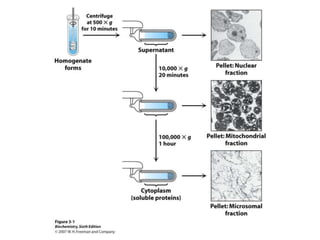
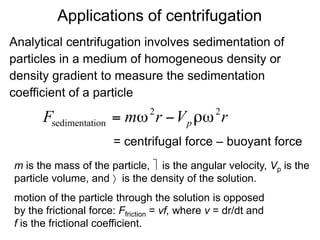
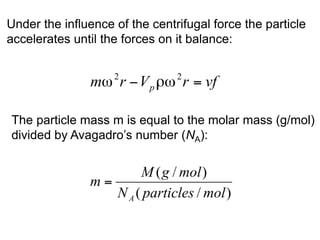
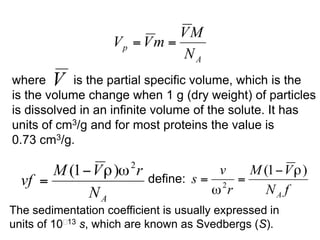
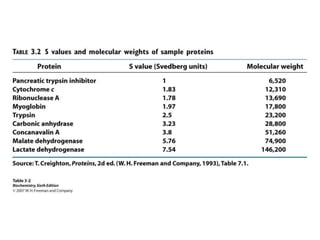
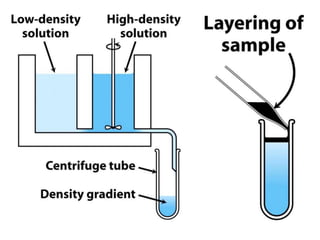
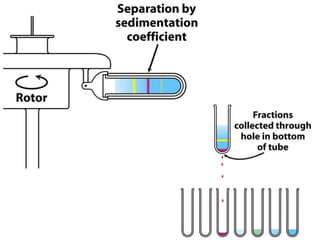
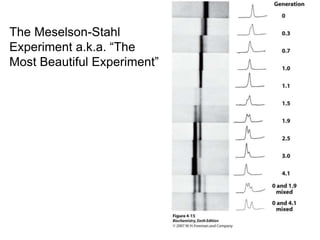
The document discusses centrifugation techniques used in biochemical research. It defines centrifugal force and relative centrifugal force (RCF). It states that to centrifuge a sample at 100,000g with an average radius of 4cm would require setting the centrifuge to over 25,000rpm. Different types of centrifuges are used for separating particles of various sizes. Centrifugation is used for preparative and analytical techniques like sedimentation analysis to determine properties of particles like sedimentation coefficients.