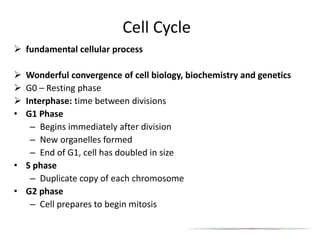
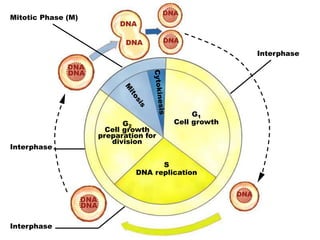
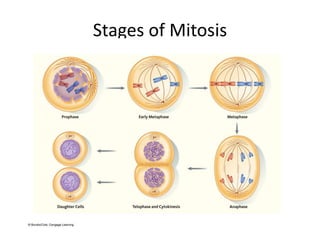
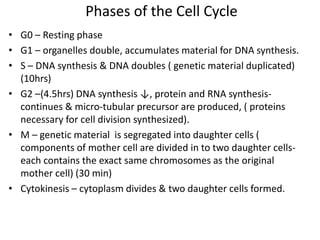
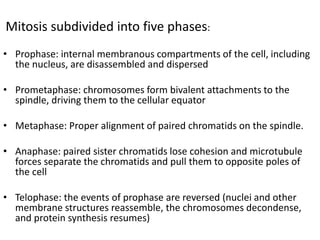
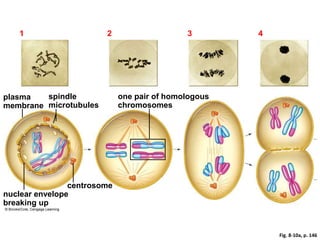
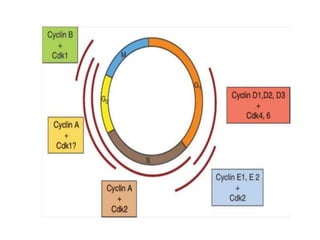
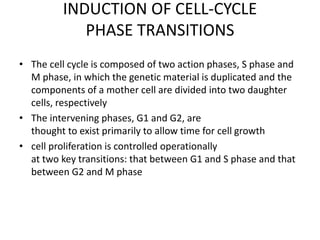
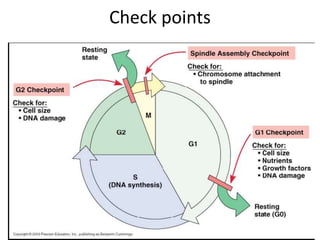
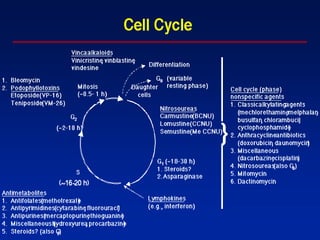
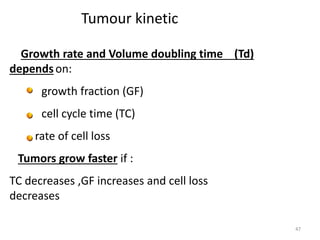
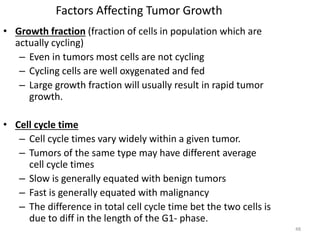
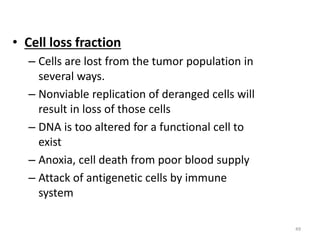
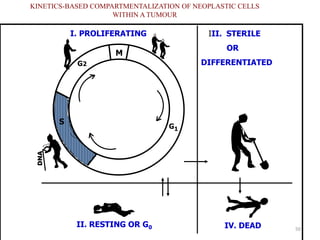
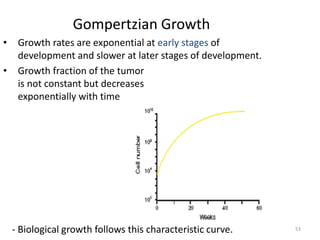
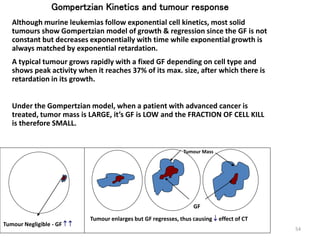
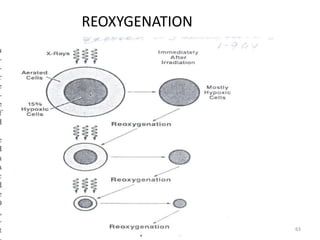
- The cell cycle consists of four main phases - G1, S, G2, and M. The G1, S, and G2 phases make up interphase.
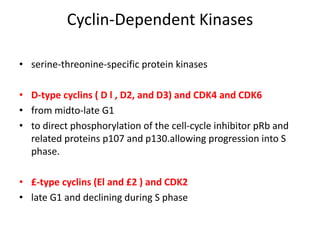
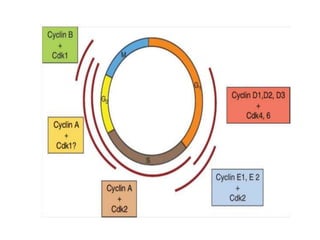
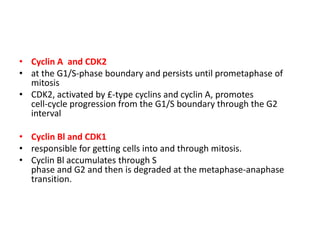
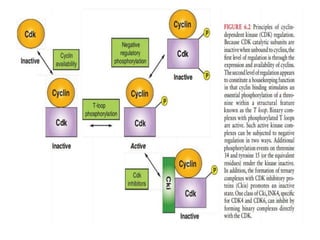
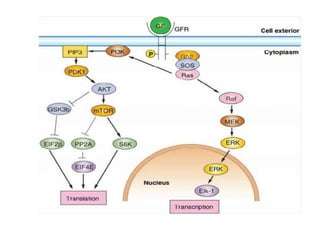
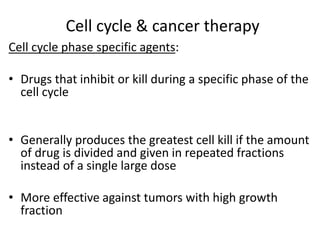
- The cell cycle is tightly regulated by cyclins and cyclin-dependent kinases (CDKs). Different cyclin-CDK complexes control progression through the different cell cycle phases.
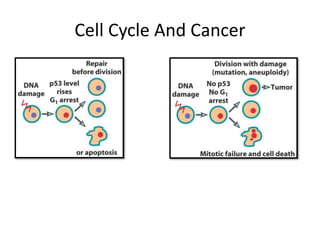
- Checkpoints exist to monitor DNA damage before progression into S phase and M phase. These checkpoints are regulated by proteins like ATM, ATR, Chk1, Chk2, and p53.
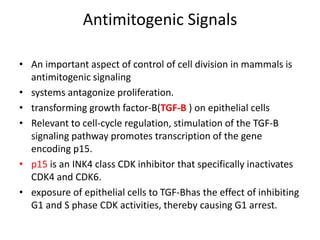
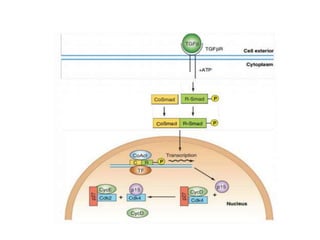
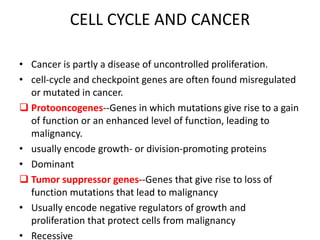
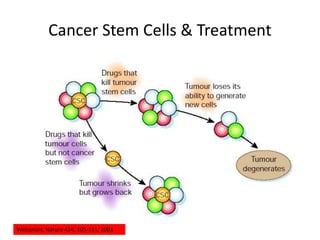
- Dysregulation of cell cycle control and checkpoint pathways contributes to uncontrolled cell proliferation in cancer. Both oncogenes and tumor