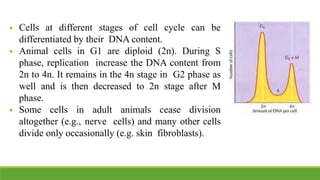
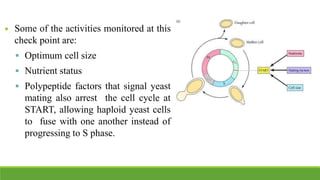
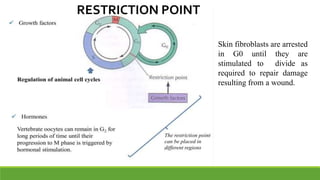
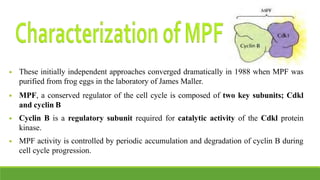
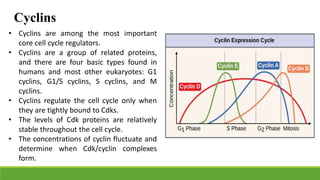
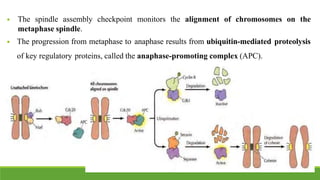
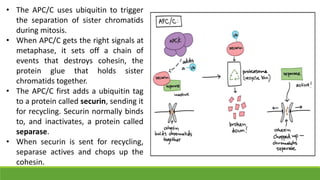
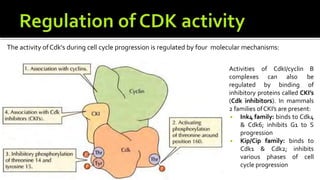
The cell cycle is tightly regulated and divided into four main phases: G1, S, G2, and M. Progression through the cell cycle is controlled by cyclins and cyclin-dependent kinases (Cdks). Cyclins activate Cdks and direct them to specific protein targets to drive cell cycle events. Key control points include the Start checkpoint in G1, the restriction point, and control of the G2 to M transition. Damage checkpoints and the spindle assembly checkpoint ensure fidelity of DNA replication and chromosome segregation. The anaphase promoting complex/cyclosome (APC/C) triggers the onset of anaphase through ubiquitin-mediated proteolysis.