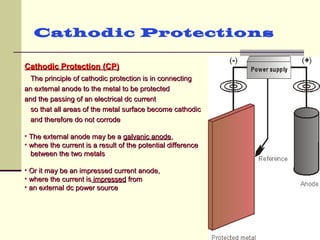
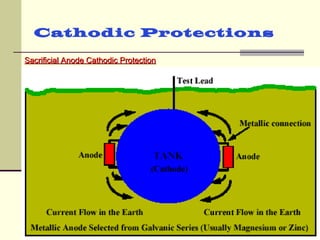
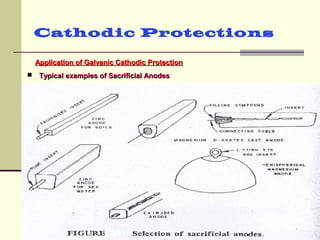
The document discusses cathodic protection systems, which are used to protect underground pipelines and storage tanks from corrosion. It describes two types of cathodic protection - galvanic (sacrificial anode) systems and impressed current systems. Galvanic systems use more electrically active sacrificial anodes to supply current, while impressed current systems use an external DC power source. The document provides details on corrosion processes, factors affecting corrosion rates, and how cathodic protection works to make protected structures cathodic to prevent corrosion.