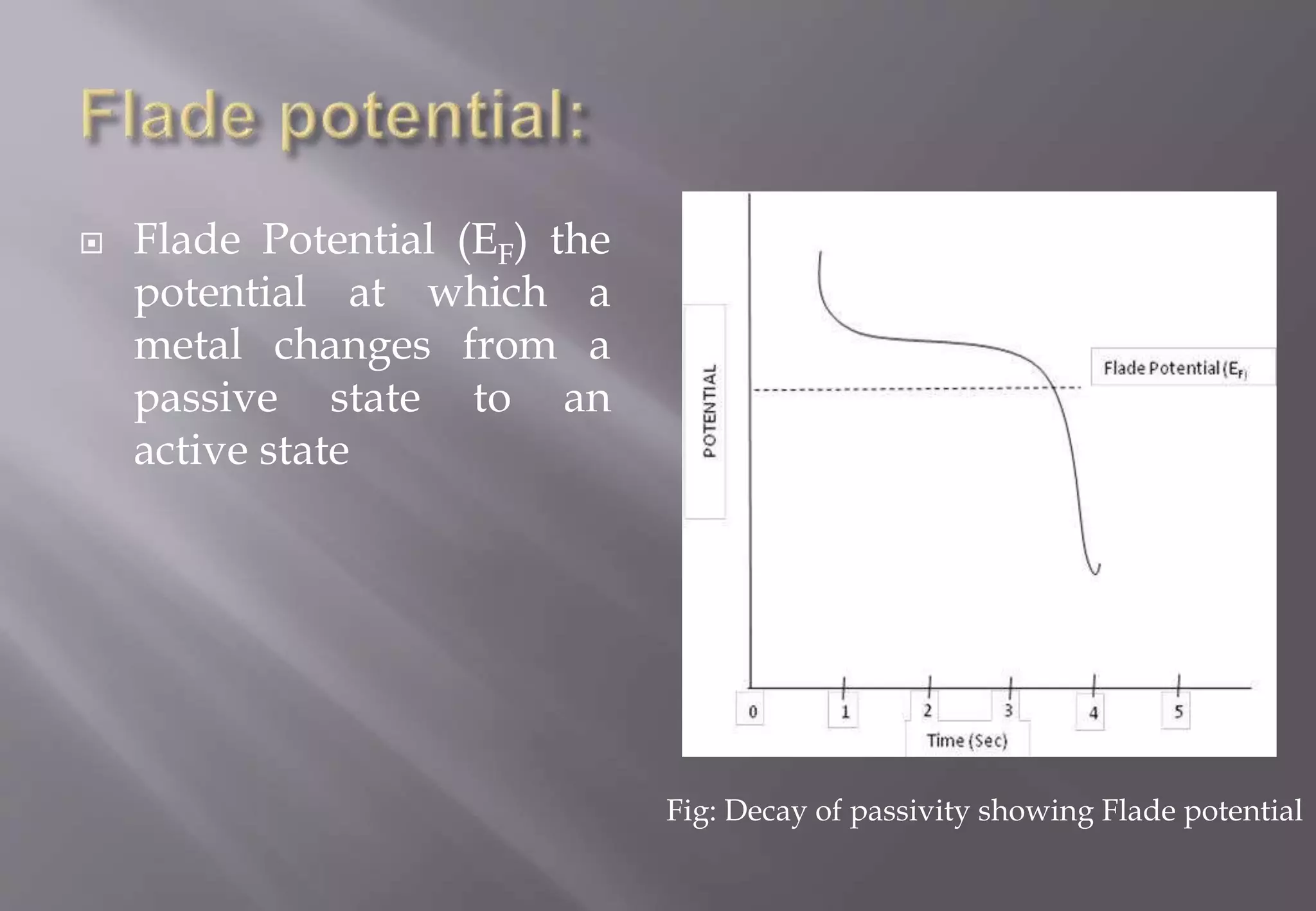
This document discusses corrosion and passivity of metals, specifically:
1) It defines passivity as the formation of a thin surface film under oxidizing conditions that provides corrosion resistance to some metals and alloys.
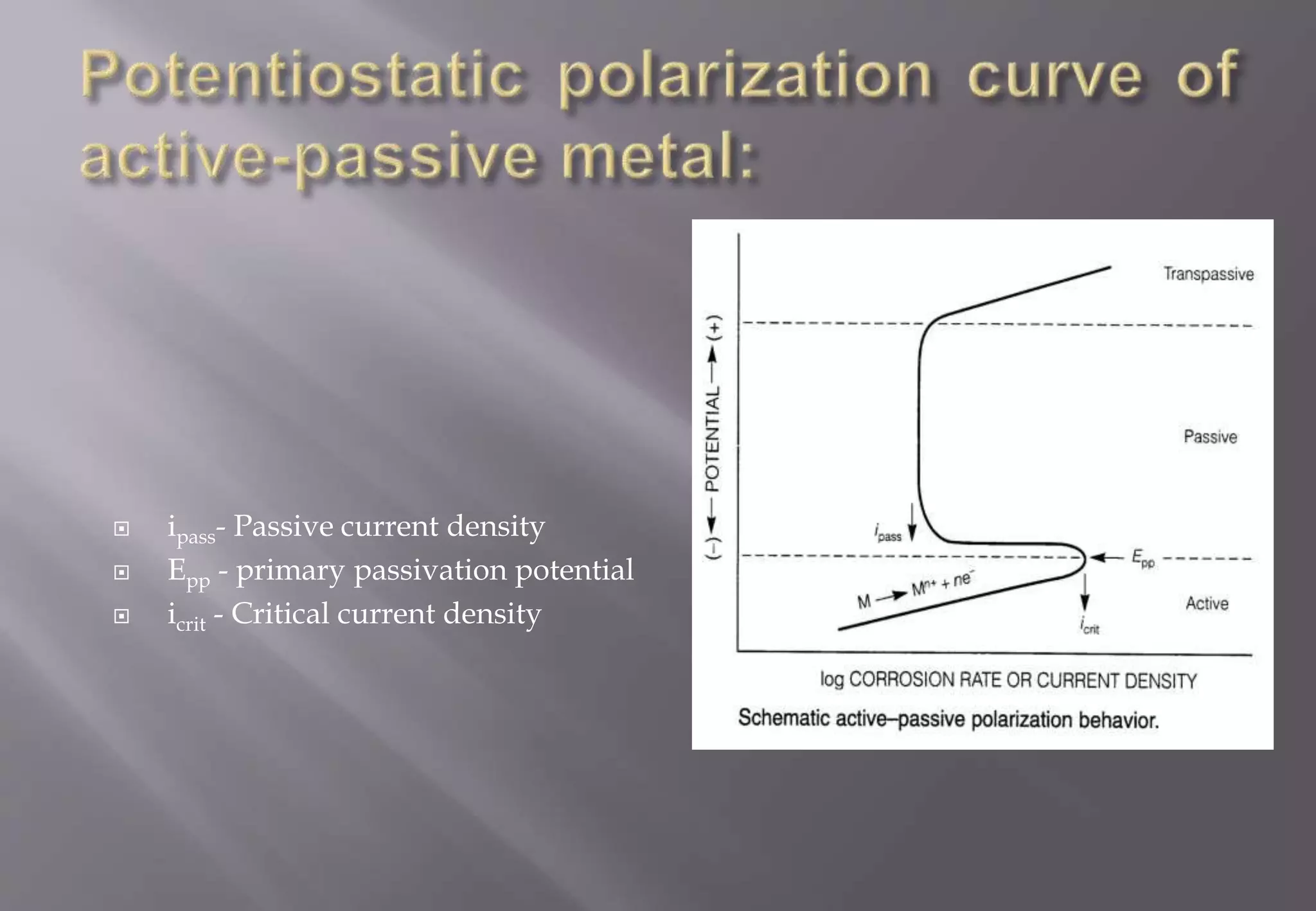
2) It describes potentiostatic polarization as a technique to control metal polarization in electrolytes to observe corrosion behaviors.
3) It lists applications such as corrosion product analysis, alloy selection, and localized corrosion analysis.
4) It discusses concepts related to passivity including passive current density, primary passivation potential, and critical current density.