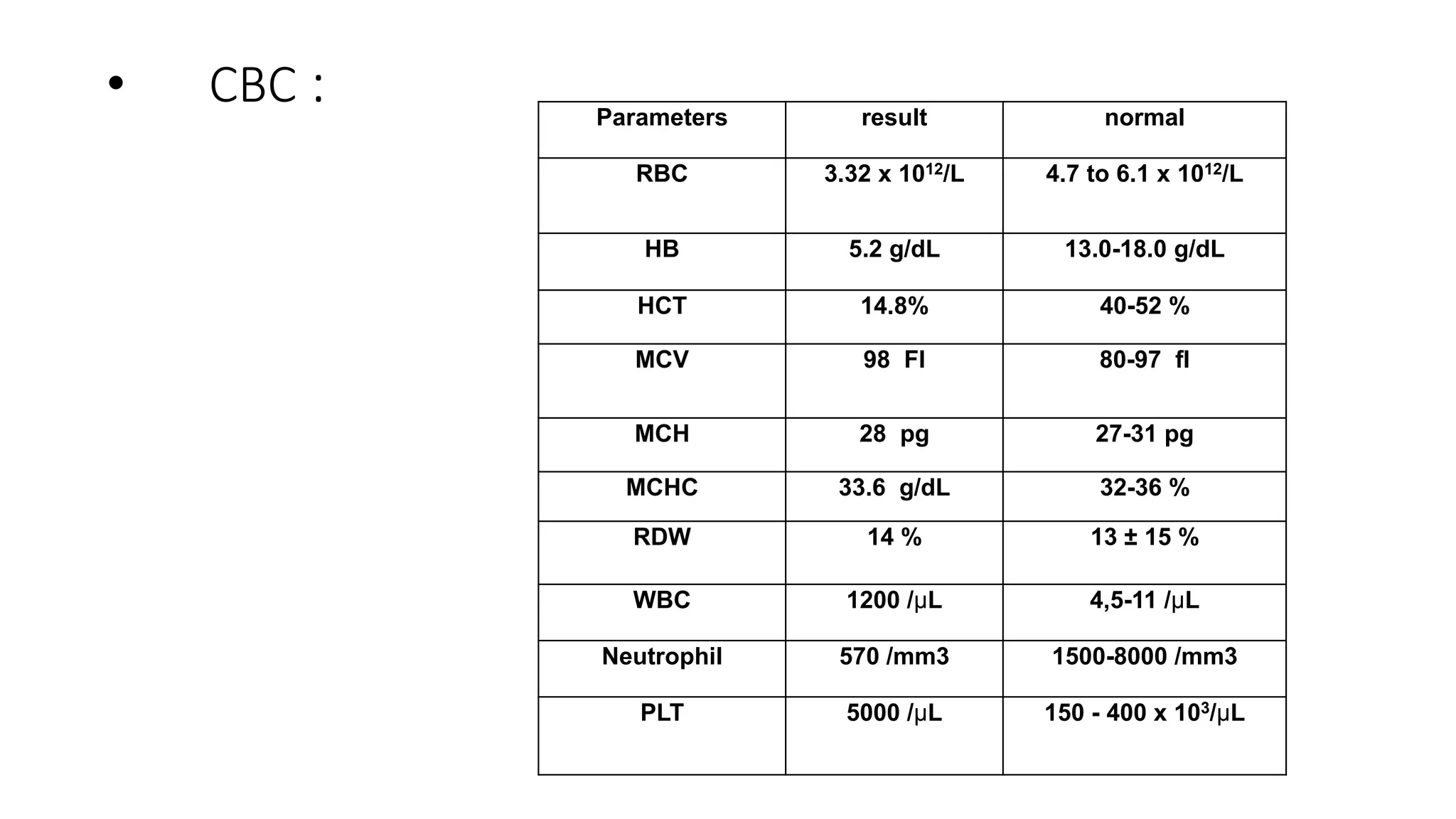
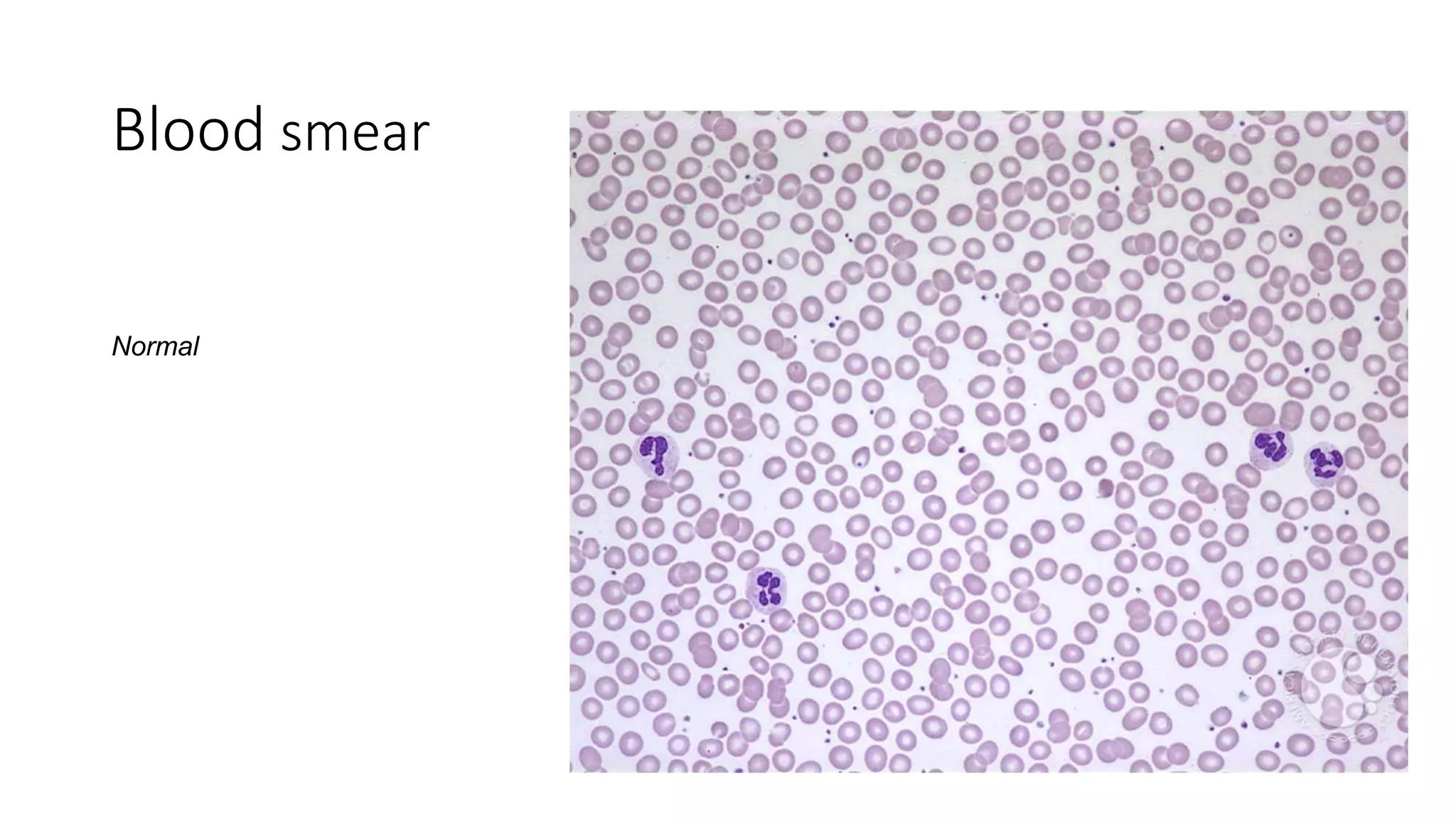
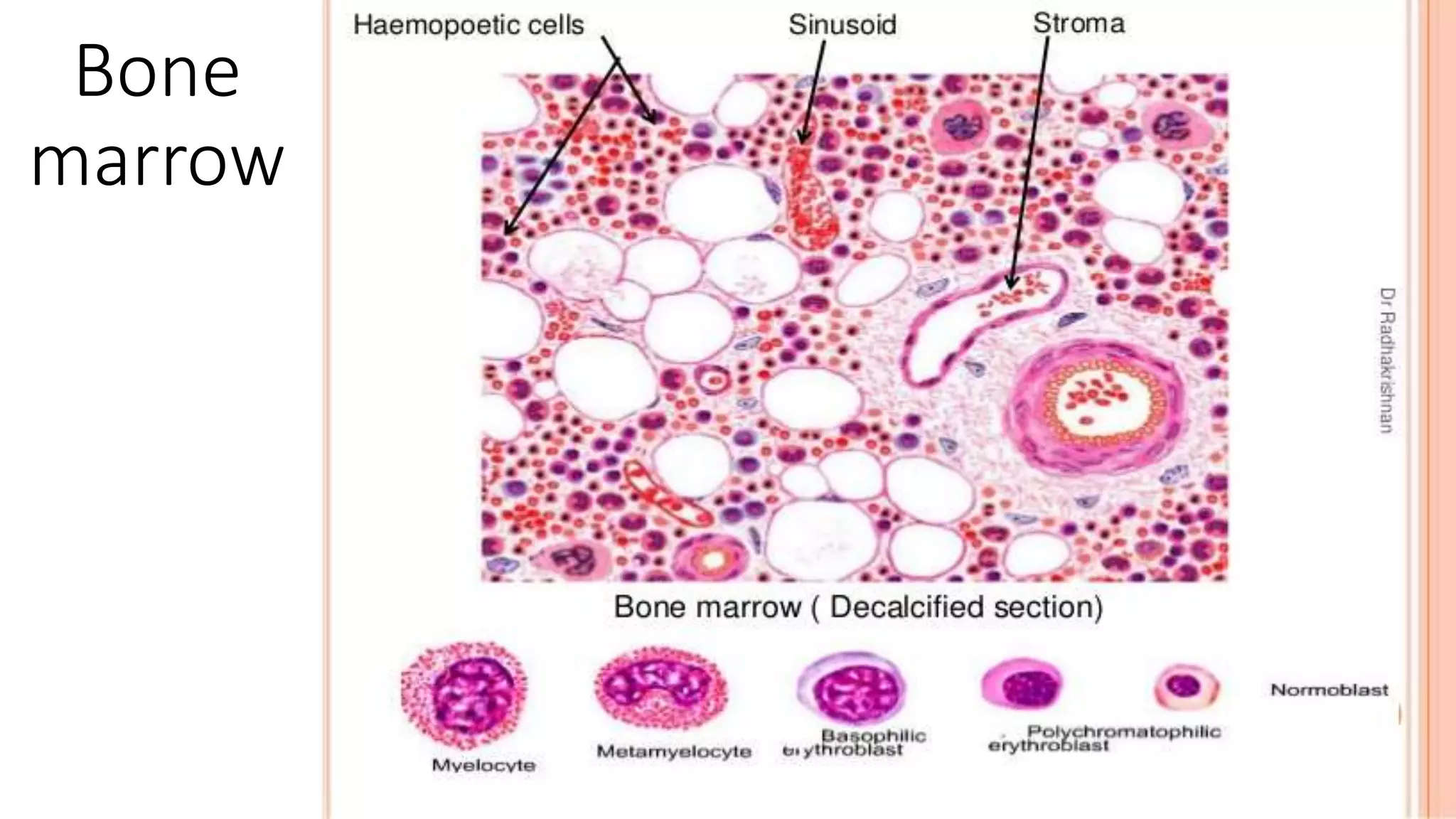
This case presentation describes a 24-year-old female patient who was admitted to the hospital with symptoms of weakness, shortness of breath, fever, and recurrent nosebleeds. Laboratory tests showed very low blood cell counts. A bone marrow examination found a markedly hypocellular marrow with few hematopoietic cells. The patient was diagnosed with aplastic anemia based on pancytopenia, low bone marrow cellularity, and no evidence of damage to stem cells or dysplasia. Aplastic anemia is a rare disease caused by damage to or decrease in bone marrow stem cells resulting in bone marrow failure and replacement with fat.