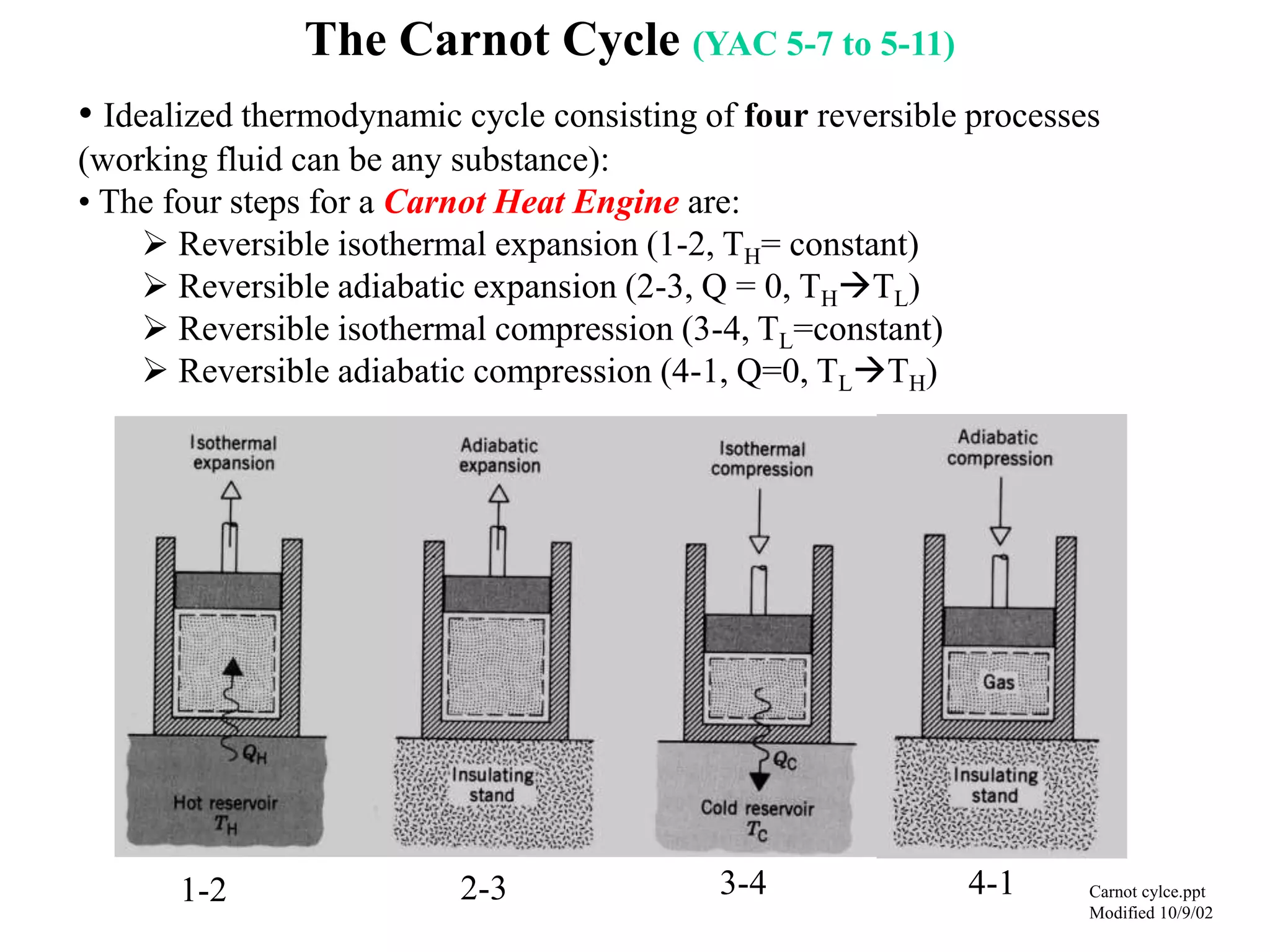
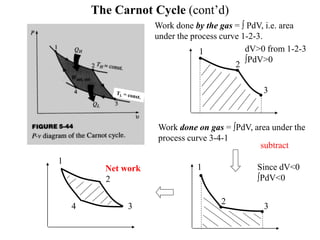
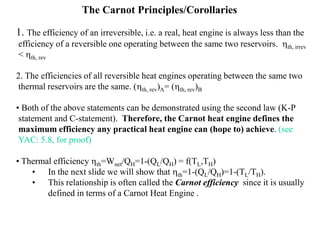
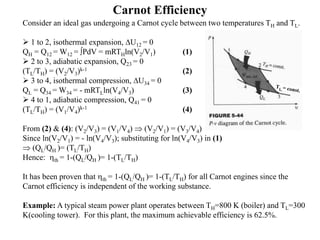
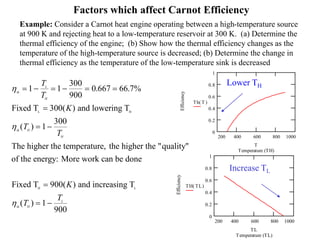
The Carnot cycle consists of four reversible processes: two isothermal expansions and two adiabatic expansions. The work done during the cycle is equal to the area under the P-V curves for the isothermal expansions, while the work done on the gas is equal to the area under the P-V curves for the adiabatic compressions. The Carnot efficiency is the maximum possible efficiency for any heat engine operating between two thermal reservoirs and is equal to 1 - (TL/TH), where TL and TH are the temperatures of the low and high reservoirs. Factors that affect the Carnot efficiency include the temperatures of the reservoirs - higher TH and lower TL result in higher efficiency.