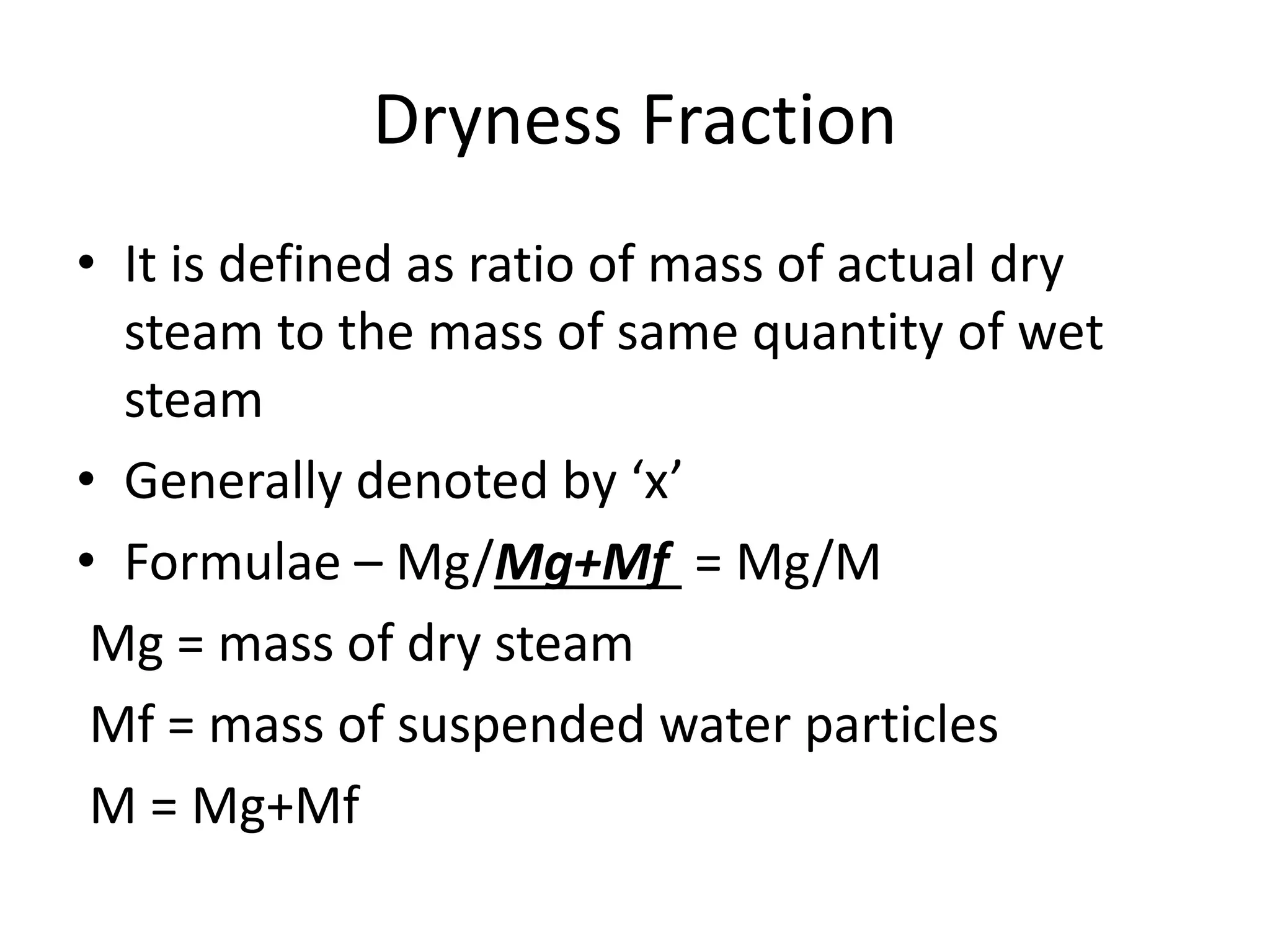
Steam is the vapor form of water and is used in steam engines and turbines. It forms when water is heated under constant pressure. As water is heated from 0°C, its temperature increases until it reaches the boiling point. At this point, further heating causes water to evaporate into steam while the temperature remains constant. This uses heat energy called the latent heat of vaporization. If heating continues, any remaining water particles evaporate, forming dry saturated steam. Dry steam can be further superheated by adding more heat at constant pressure. The temperature and pressure affect the boiling/saturation point, with higher pressure requiring more heat to reach the same temperature. Superheated steam provides benefits like more power and increased thermal efficiency.