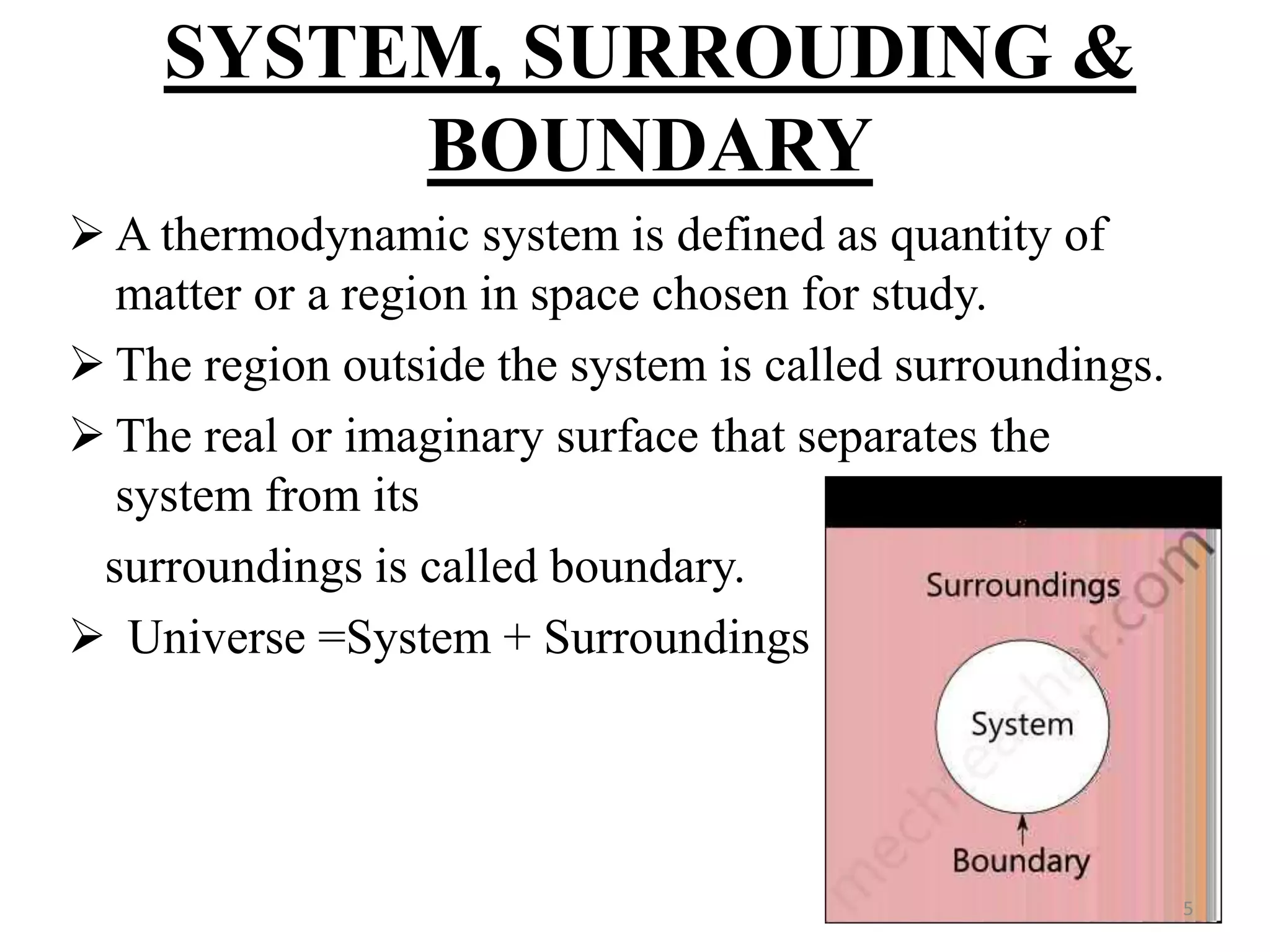
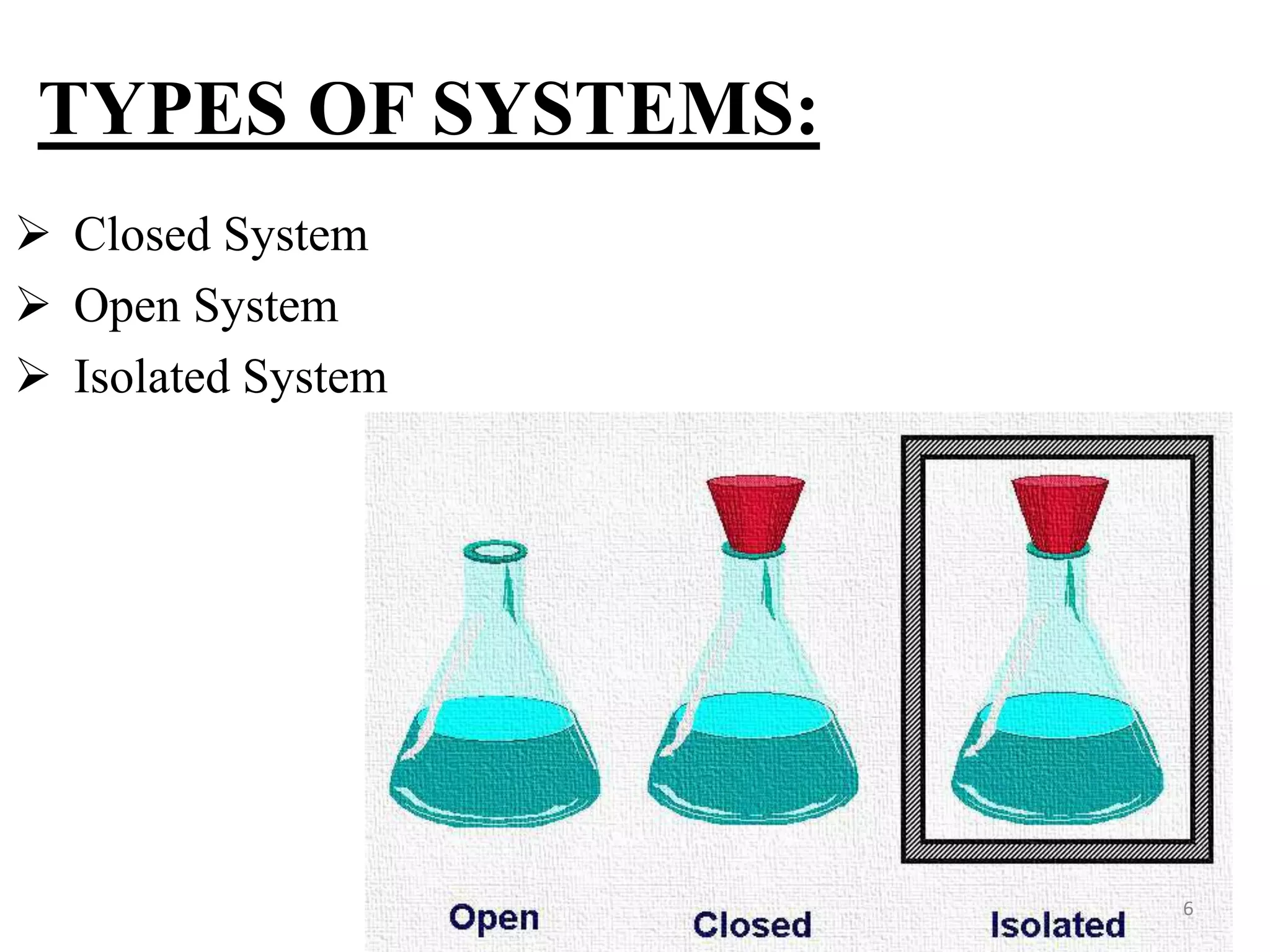
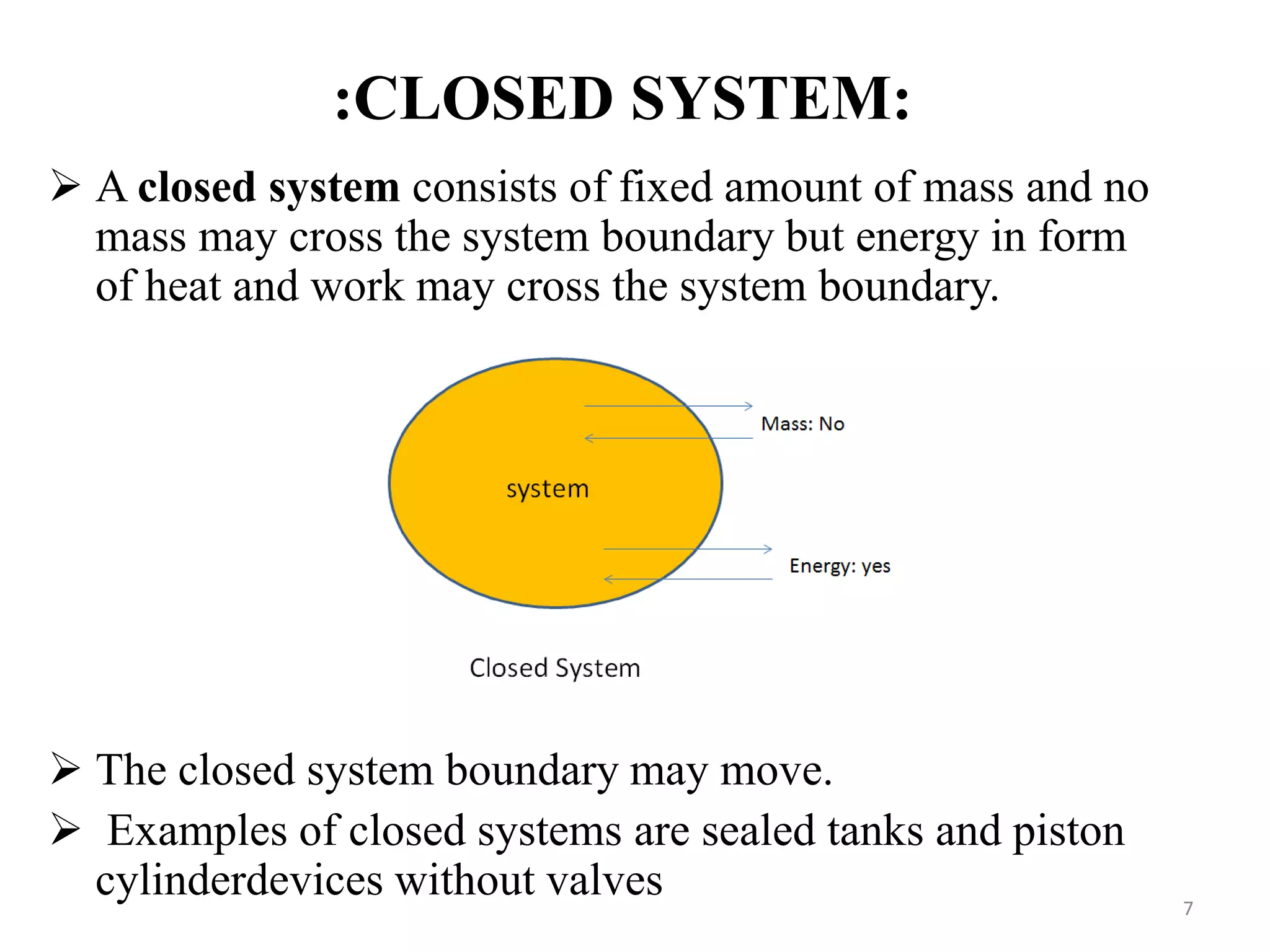
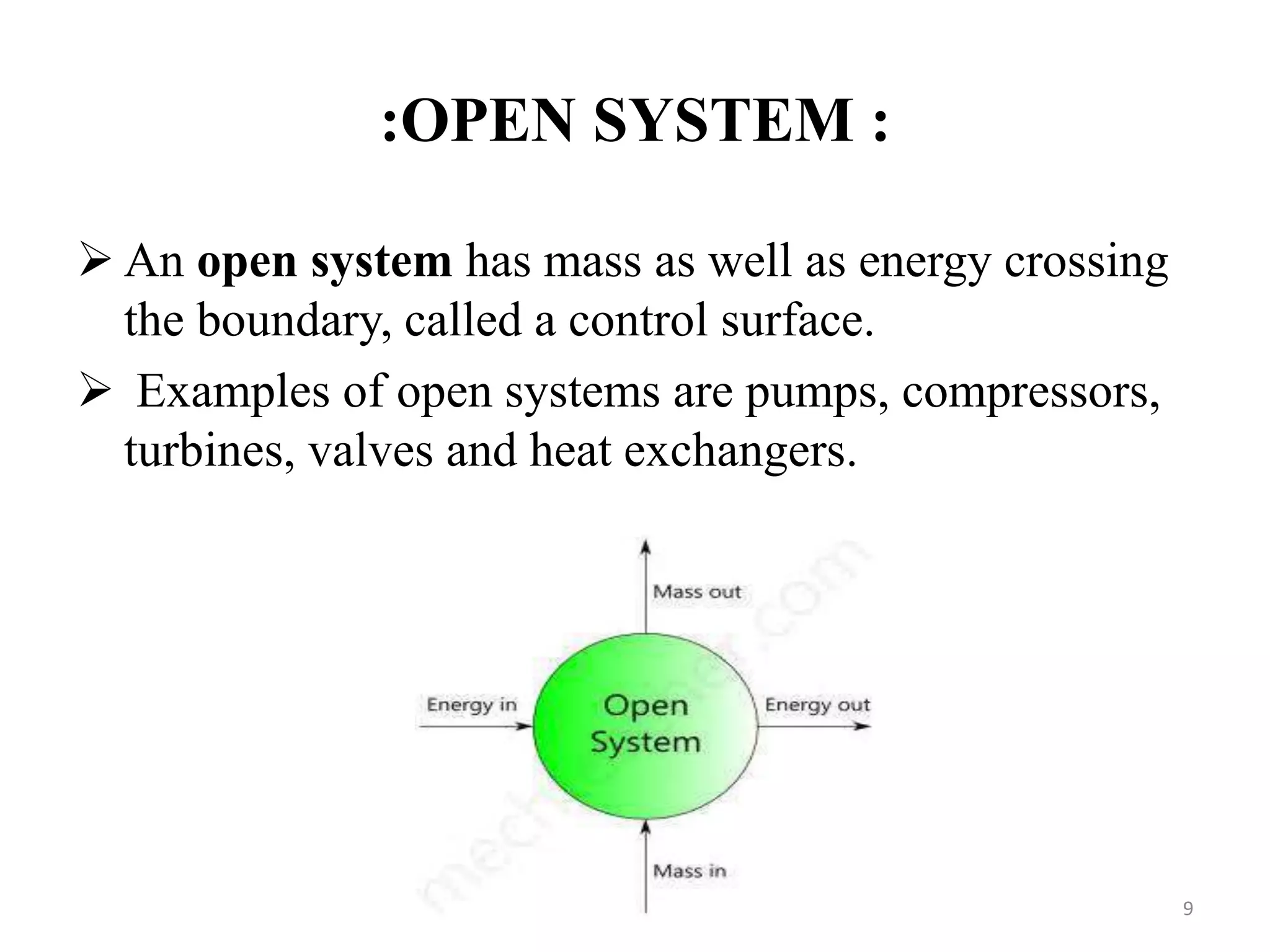
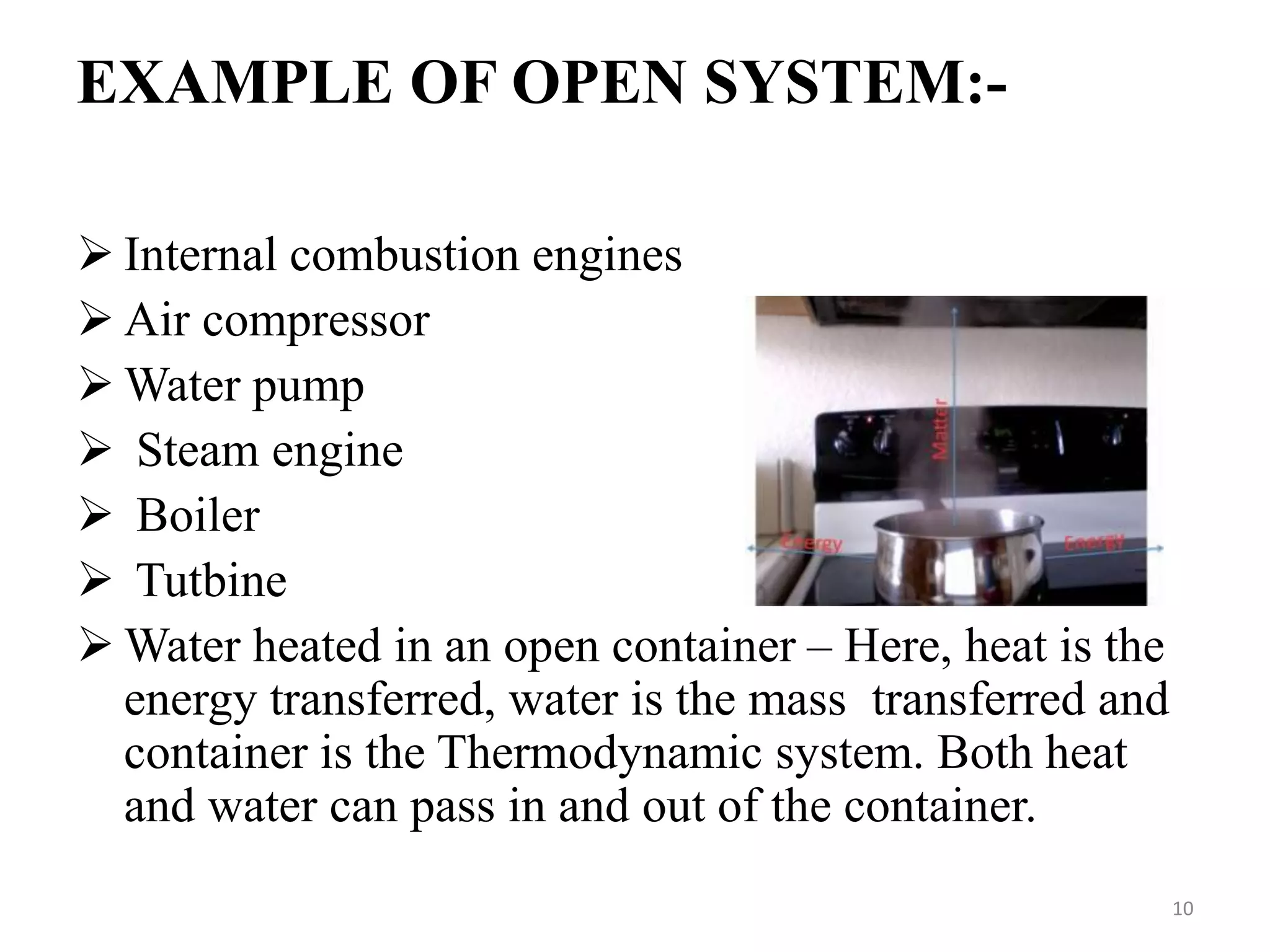
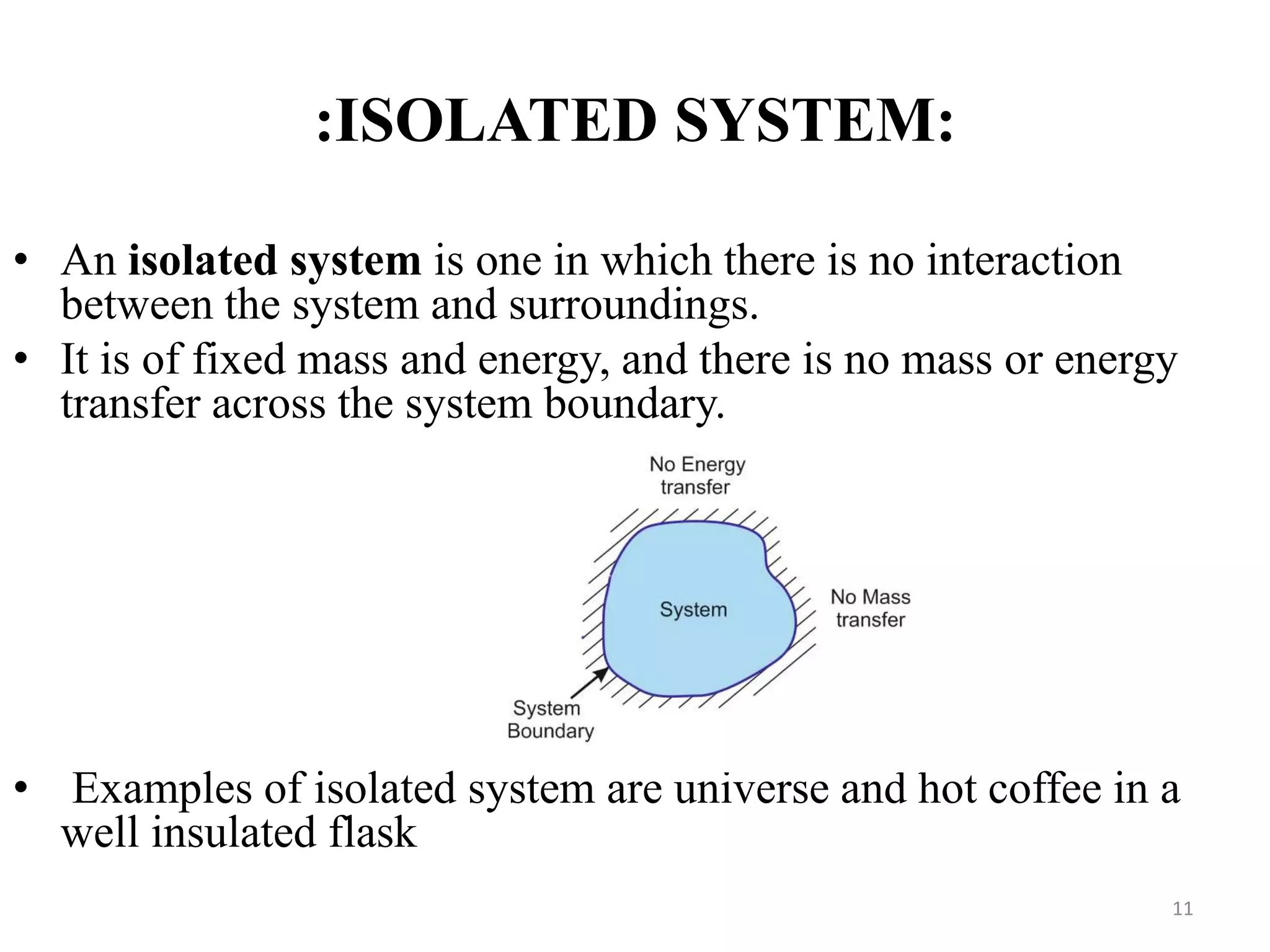
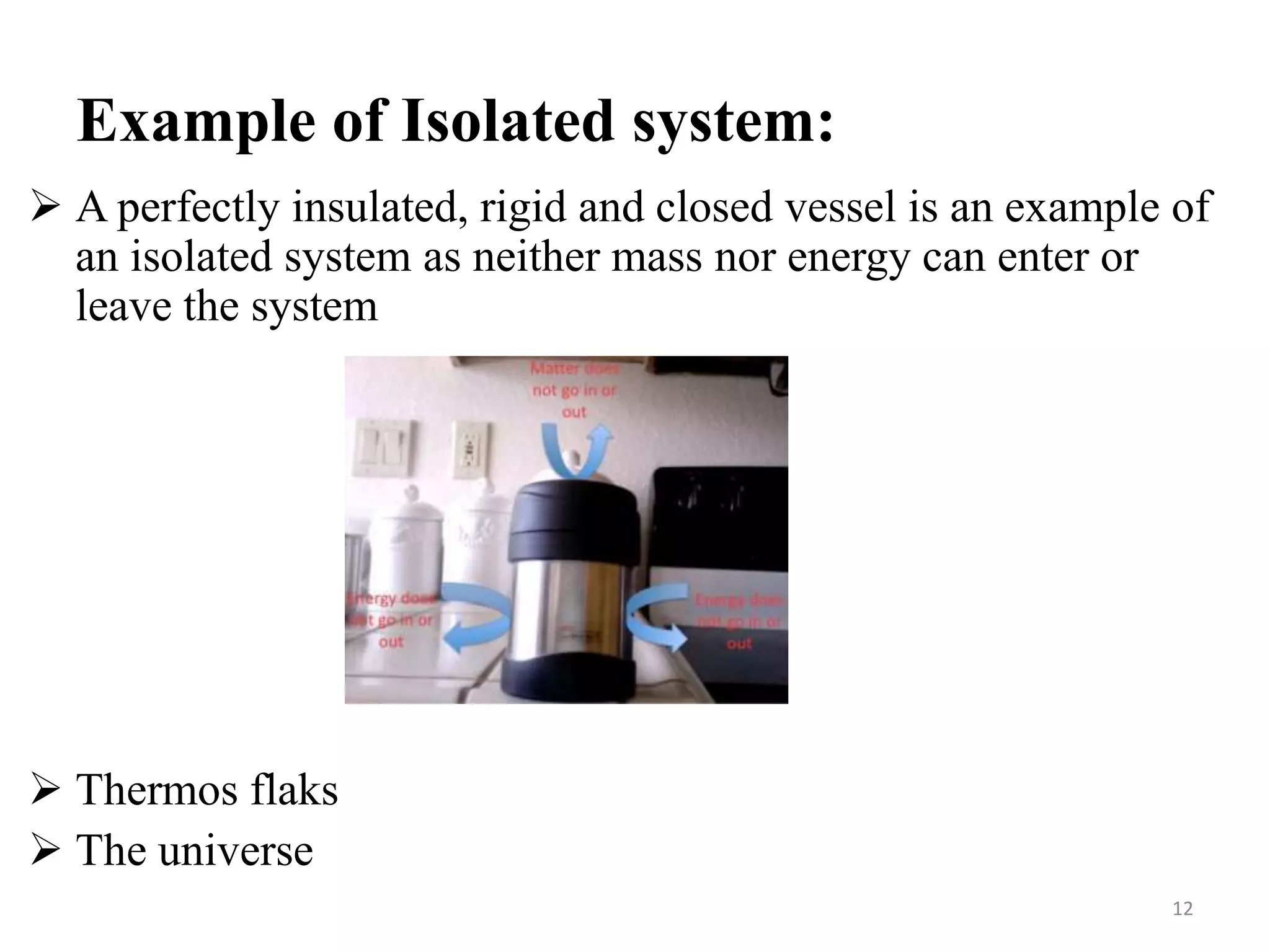
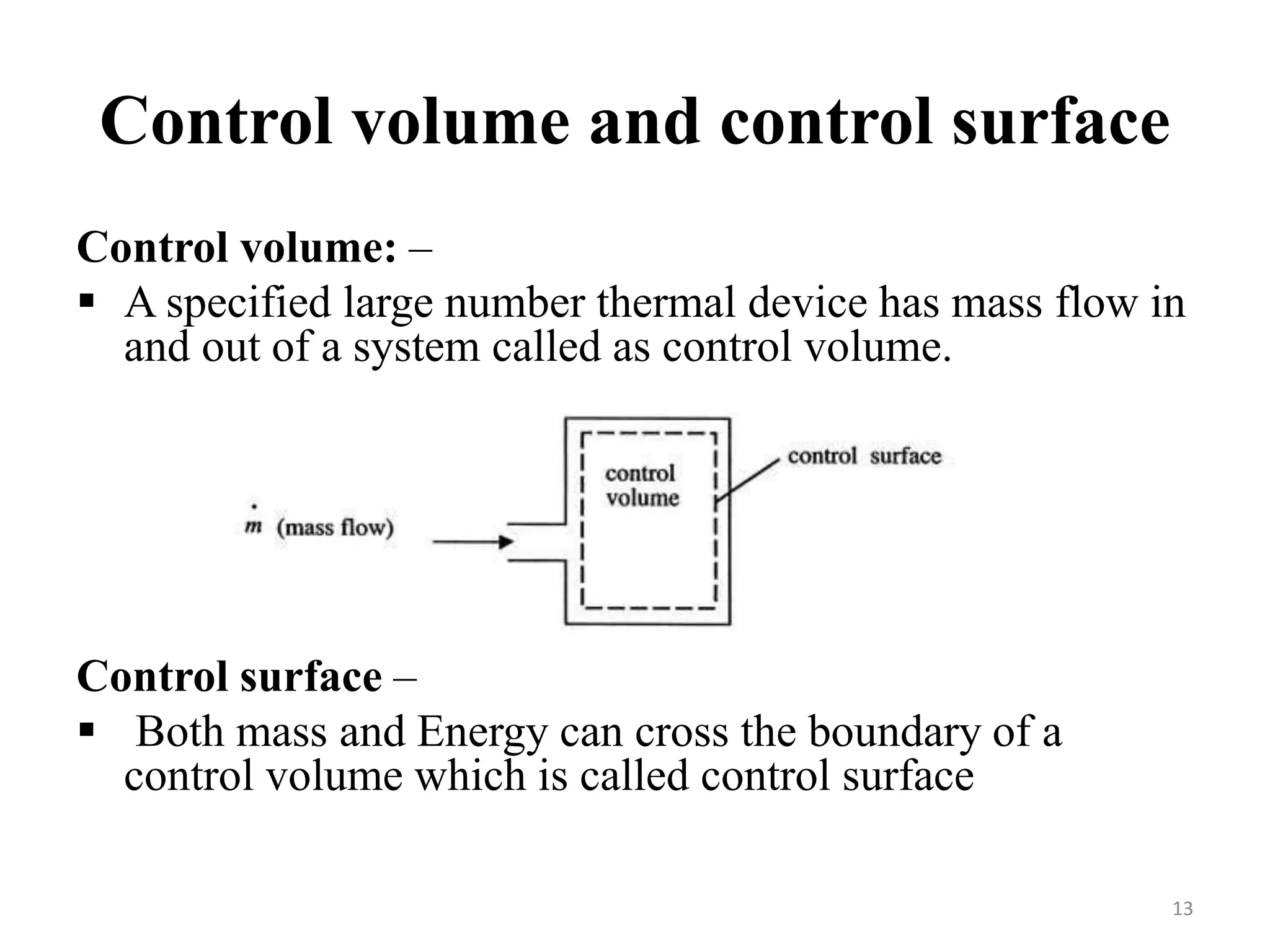
The document discusses the principles of thermodynamics from both microscopic and macroscopic perspectives, explaining the differences between classical thermodynamics and statistical approaches. It outlines types of thermodynamic systems including closed, open, and isolated systems, along with their properties and examples. Additionally, it defines properties of a thermodynamic system and distinguishes between intensive and extensive properties.