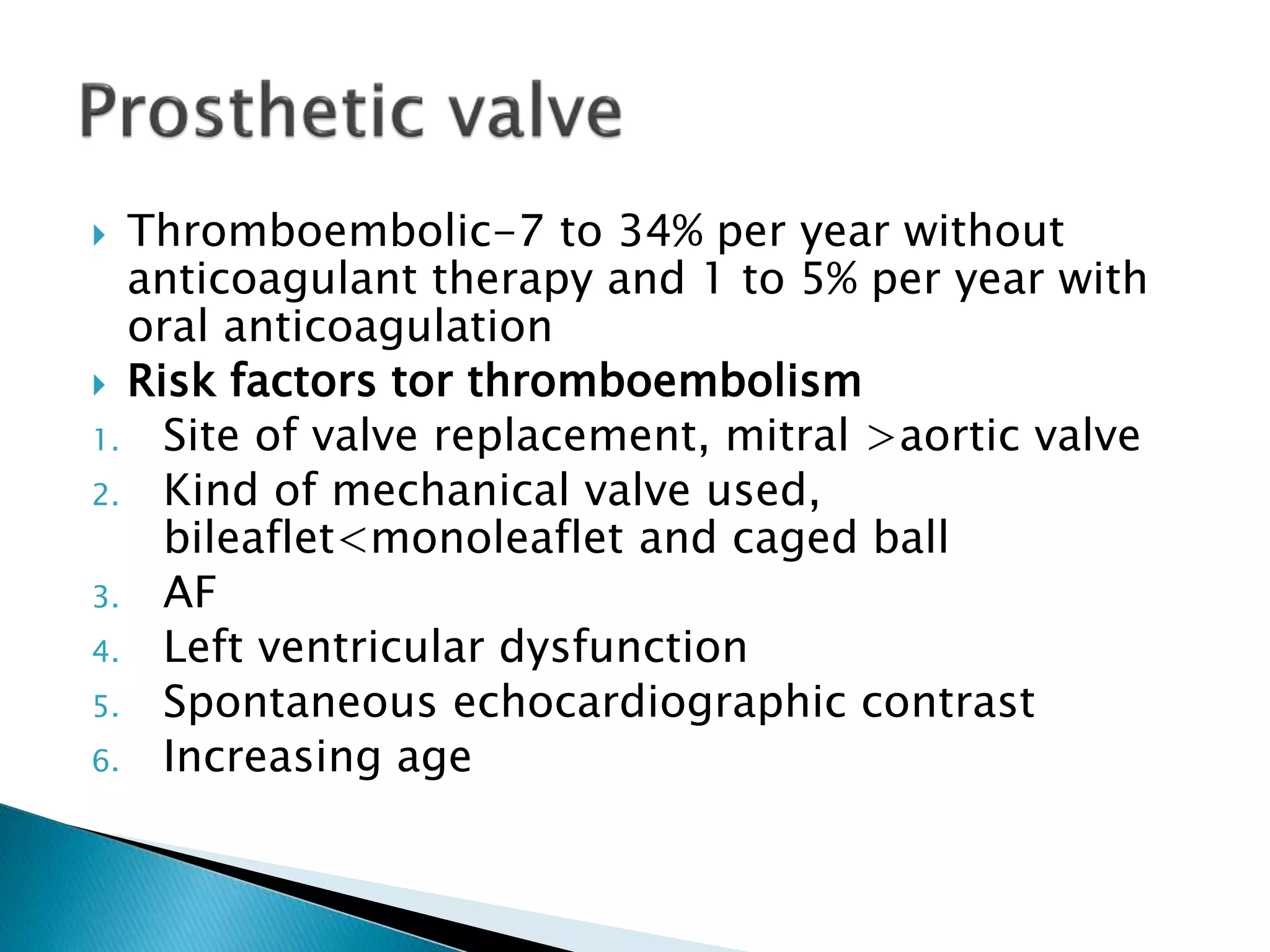
This document discusses cardioembolic stroke, which occurs when heart issues cause materials to enter the brain's blood vessels. Common causes include atrial fibrillation, heart failure, and mechanical heart valves. Diagnosis involves echocardiography and monitoring for embolic signals. Treatment depends on the specific heart condition but often includes anticoagulants to prevent clots. Anticoagulation reduces stroke risk from atrial fibrillation by 60-90% compared to placebo. Managing cardioembolic stroke risk requires identifying the underlying heart condition and addressing it with medications, surgery, or lifestyle changes.