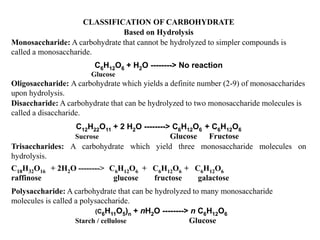
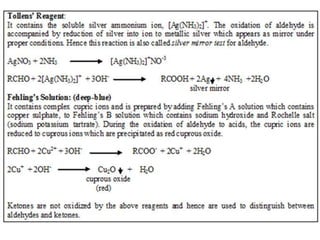
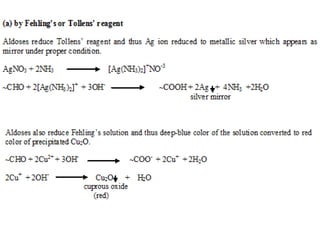
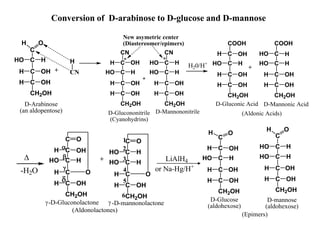
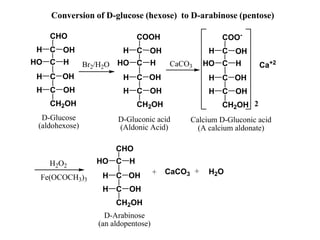
Carbohydrates can be classified based on their structure and whether they are reducing or non-reducing. The key classes are:
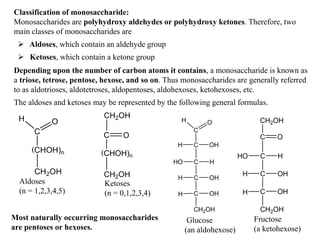
1) Monosaccharides cannot be further broken down and include aldoses and ketoses containing an aldehyde or ketone group.
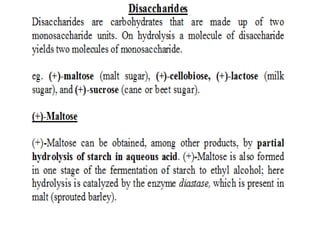
2) Disaccharides break down into two monosaccharides like sucrose into glucose and fructose.
3) Polysaccharides break down into many monosaccharide units like starch into glucose.
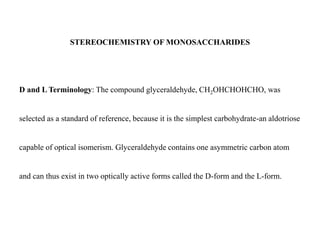
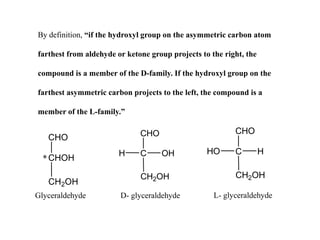
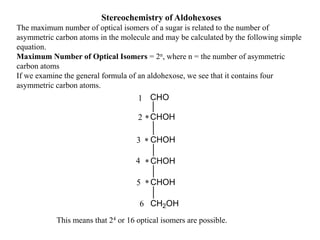
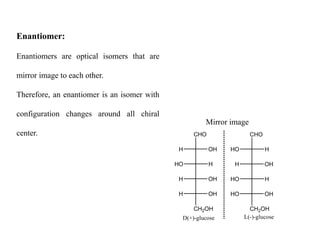
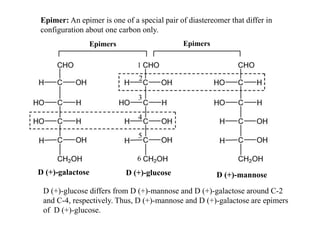
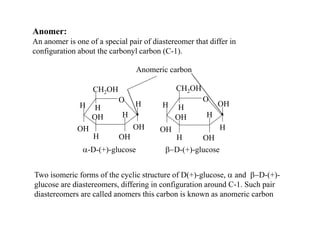
Carbohydrates exhibit stereoisomerism based on their chiral carbon atoms. Monosaccharides have D and L forms that are enantiomers, while epimers differ at one carbon. Anomers of cyclic forms