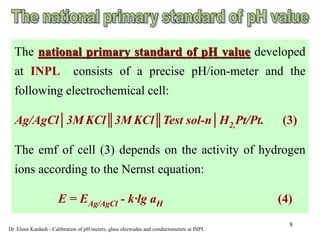
This document discusses calibration services provided by Dr. Elena Kardash at the National Physical Laboratory of Israel (INPL). INPL has developed national standards for pH and electrolytic conductivity that allow for traceable calibration of pH meters, glass electrodes, conductometers, and other equipment. Dr. Kardash offers on-site calibration of these devices using INPL's standards to ensure measurements can be tied directly to Israel's national measurement standards. Calibration procedures involve comparing the readings from a device to INPL's primary standards under controlled conditions.