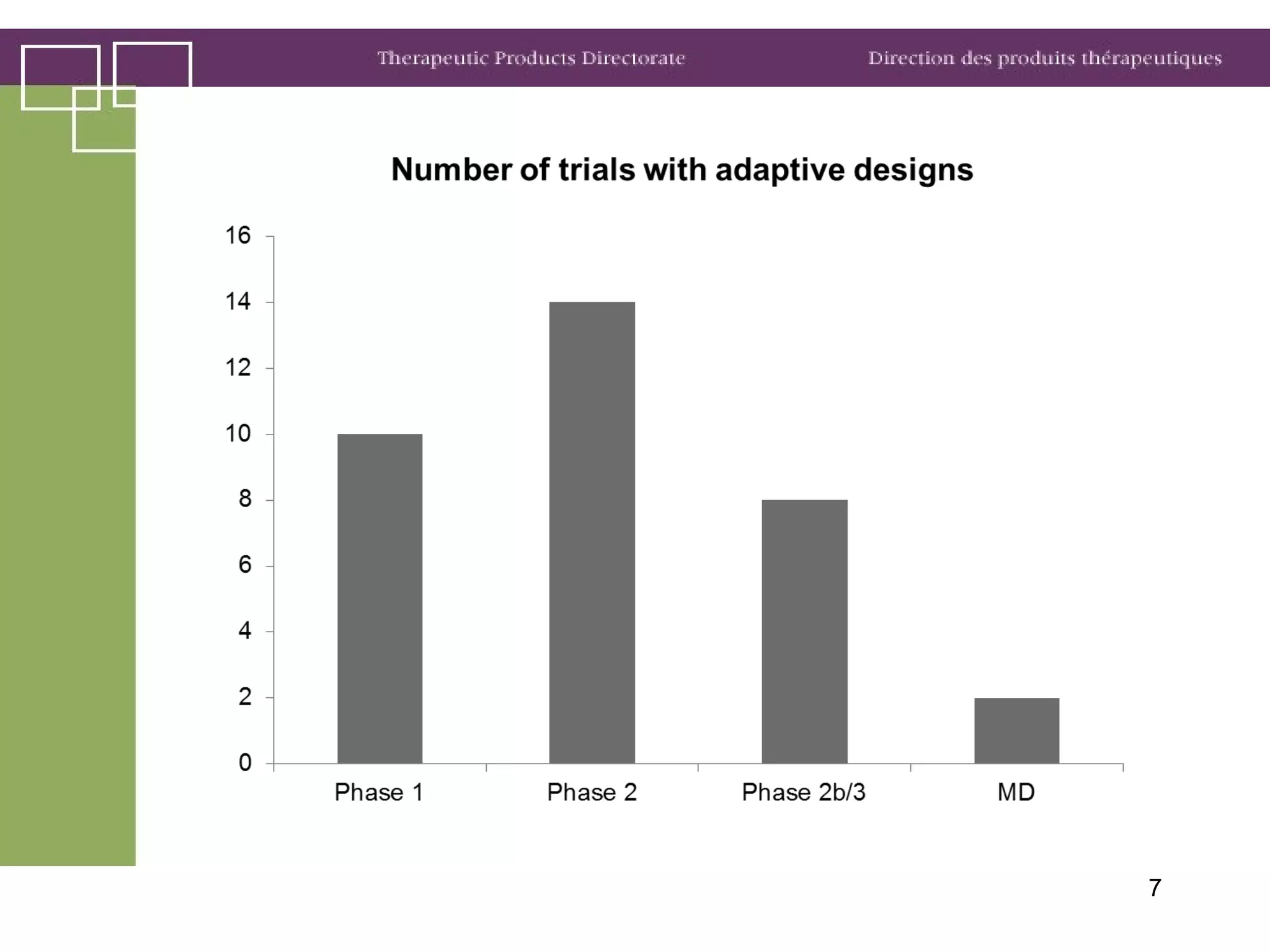
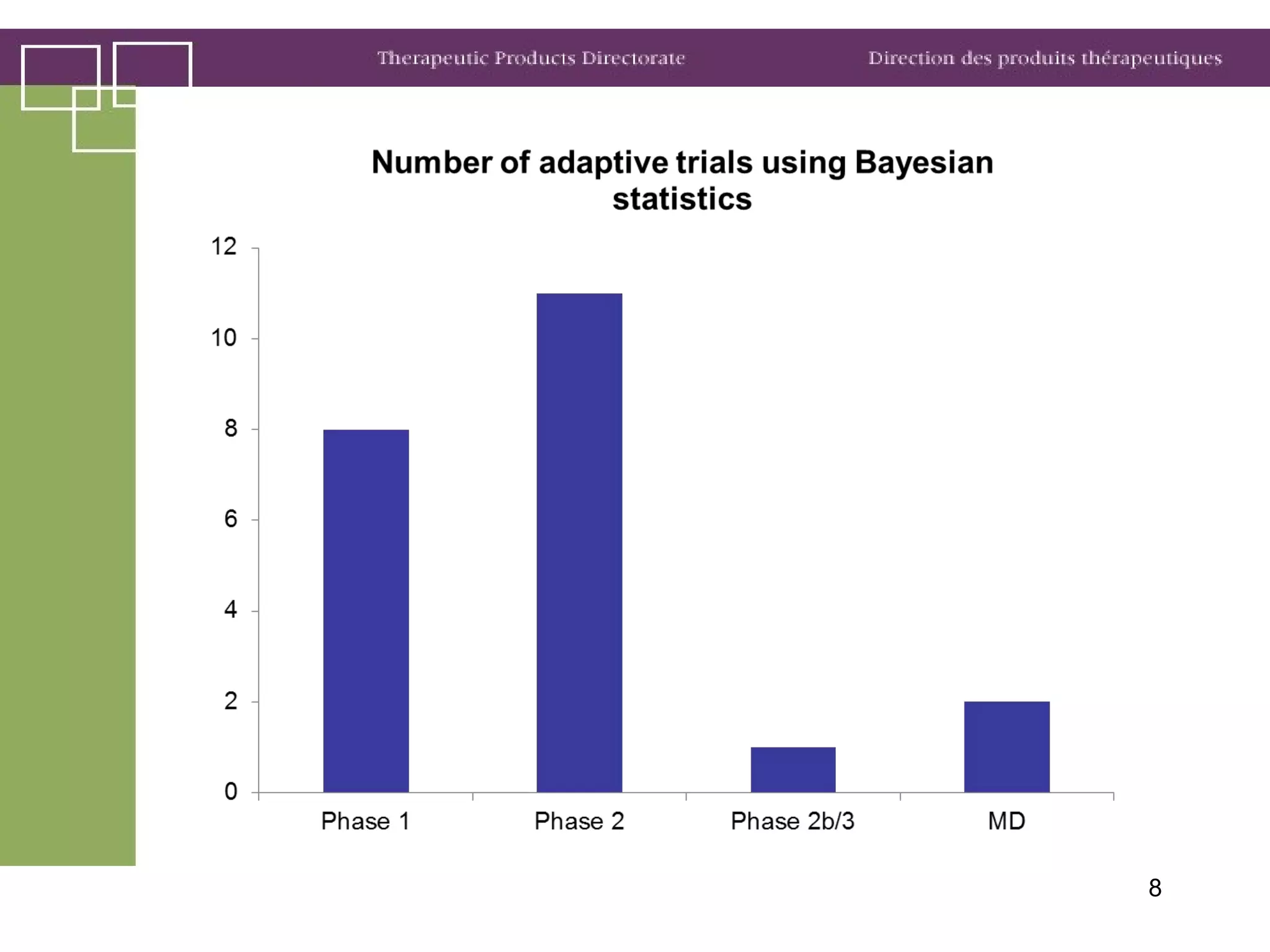
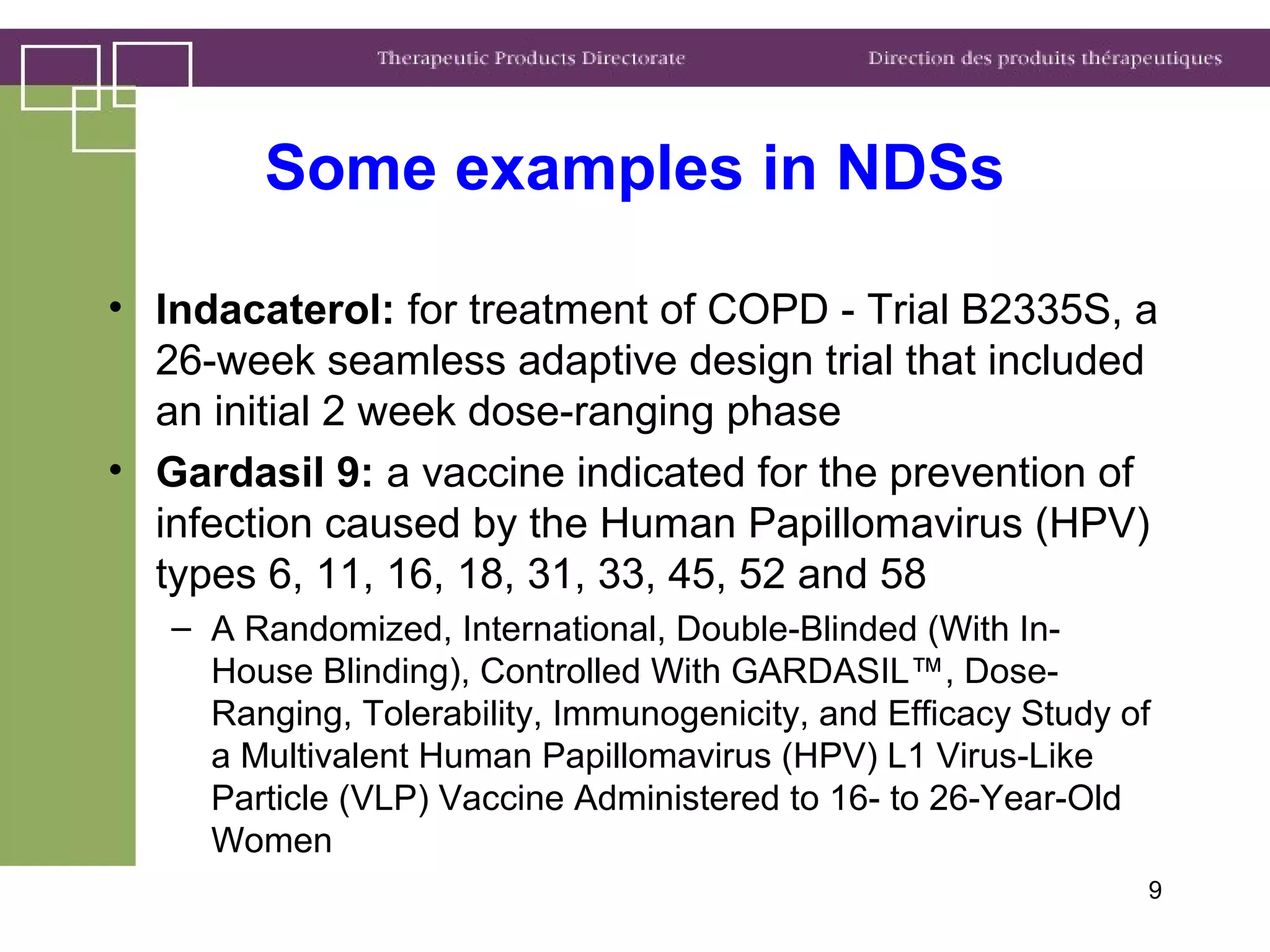
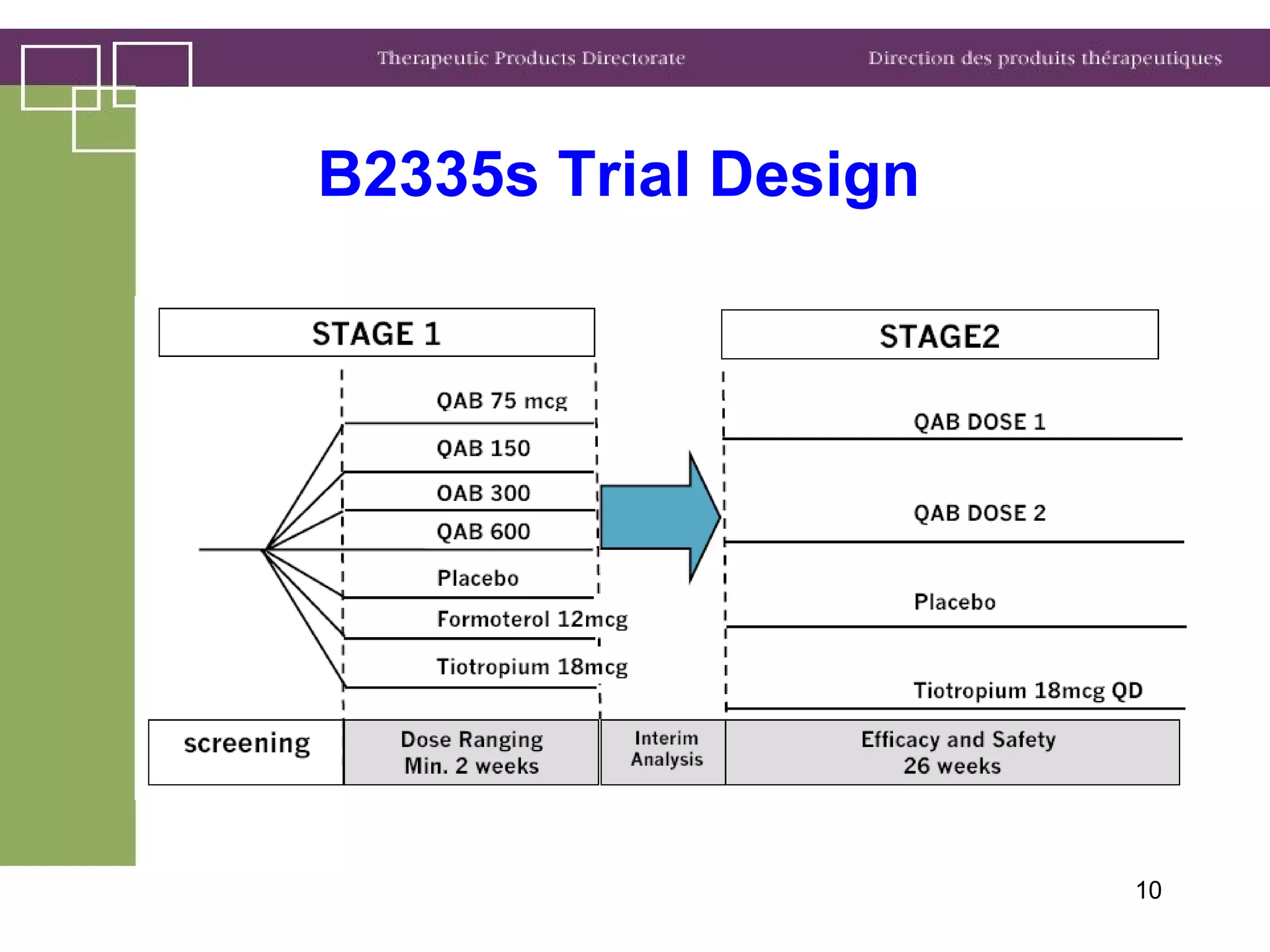
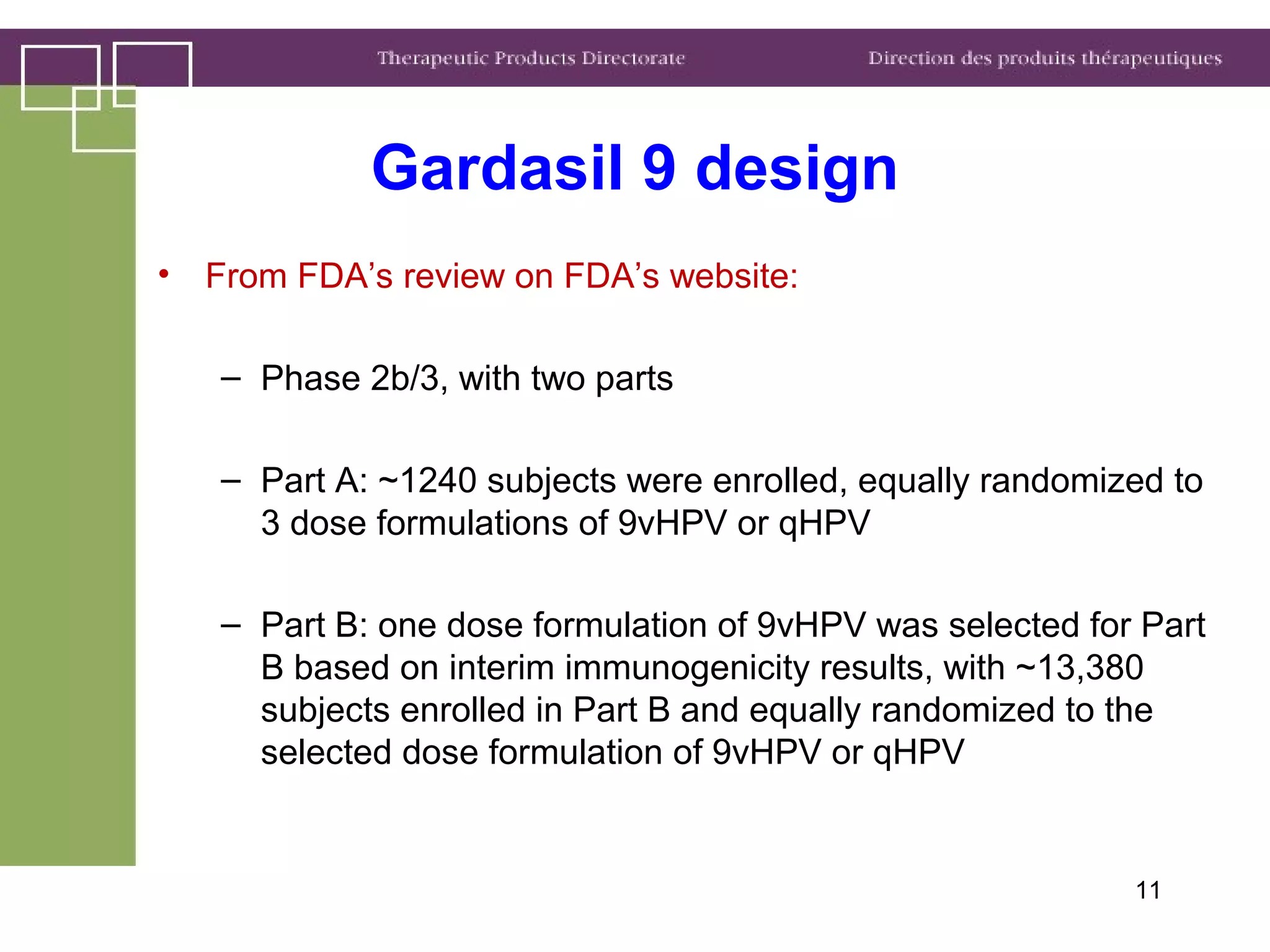
Health Canada regulates clinical trials and drug approvals. Adaptive clinical trial designs are increasing and can modify aspects of ongoing trials using accumulating data. Health Canada's main concerns are appropriate scientific questions, error control, and avoiding bias. Examples seen include adaptive randomization, sample size changes, and seamless phase 2/3 designs. Case studies demonstrate increased complexity, new statistical methods, and more simulation use. Health Canada recommends early consultation and education on adaptive designs.