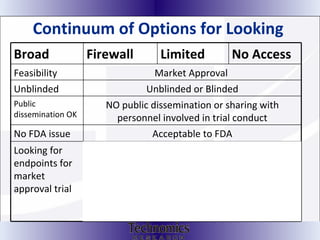
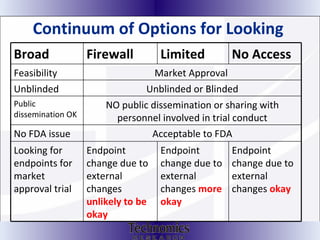
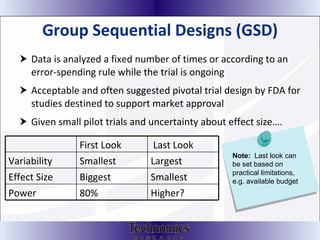
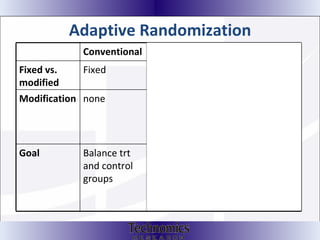
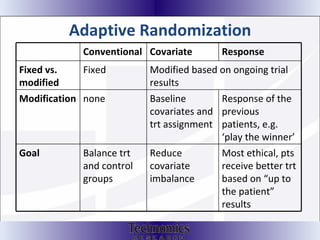
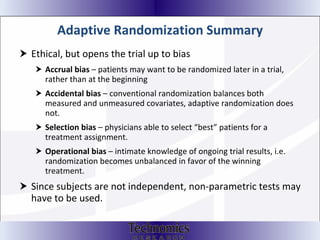
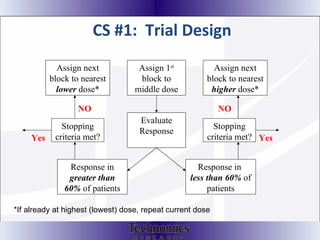
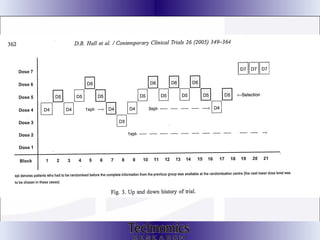
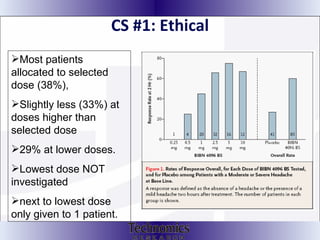
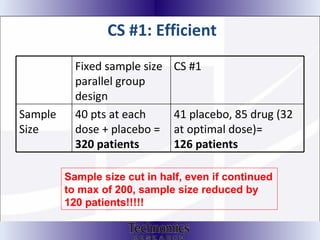
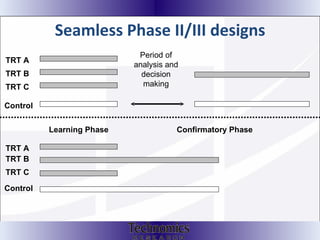
The document summarizes adaptive trial designs, where accumulating data is used to modify aspects of an ongoing study without undermining its validity. It discusses sample size re-estimation, group sequential designs, adaptive randomization, and adaptive dose escalation to make trials more efficient and ethical. Seamless phase II/III designs that combine learning and confirmation phases into one protocol are also described. The FDA accepts some adaptive designs for approval but is cautious of other novel approaches. Case studies demonstrate how adaptations can substantially reduce sample sizes.




![Adaptive Designs are Any design that uses accumulating data to decide how to modify aspects of the study as it continues, without undermining the validity and integrity of the trial. Changes are made by “by design” [prospectively] and not on an ad hoc basis ; Adaptation is not a remedy for inadequate planning .](https://image.slidesharecdn.com/introtoadaptivedesign1-12445690074-phpapp02/85/Intro-To-Adaptive-Design-5-320.jpg)


























![Thank you! Teresa Nelson, MS 218-463-5627 [email_address] www.technomicsresearch.com Ryan Wilson 612-234-8498 [email_address] www.symbiosclinical.com](https://image.slidesharecdn.com/introtoadaptivedesign1-12445690074-phpapp02/85/Intro-To-Adaptive-Design-53-320.jpg)
![If interested in contracting our services please contact: Kim Martinson Vice President-Business Development Email: [email_address] Ph: 218-331-2272 www.TechnomicsResearch.com](https://image.slidesharecdn.com/introtoadaptivedesign1-12445690074-phpapp02/85/Intro-To-Adaptive-Design-54-320.jpg)