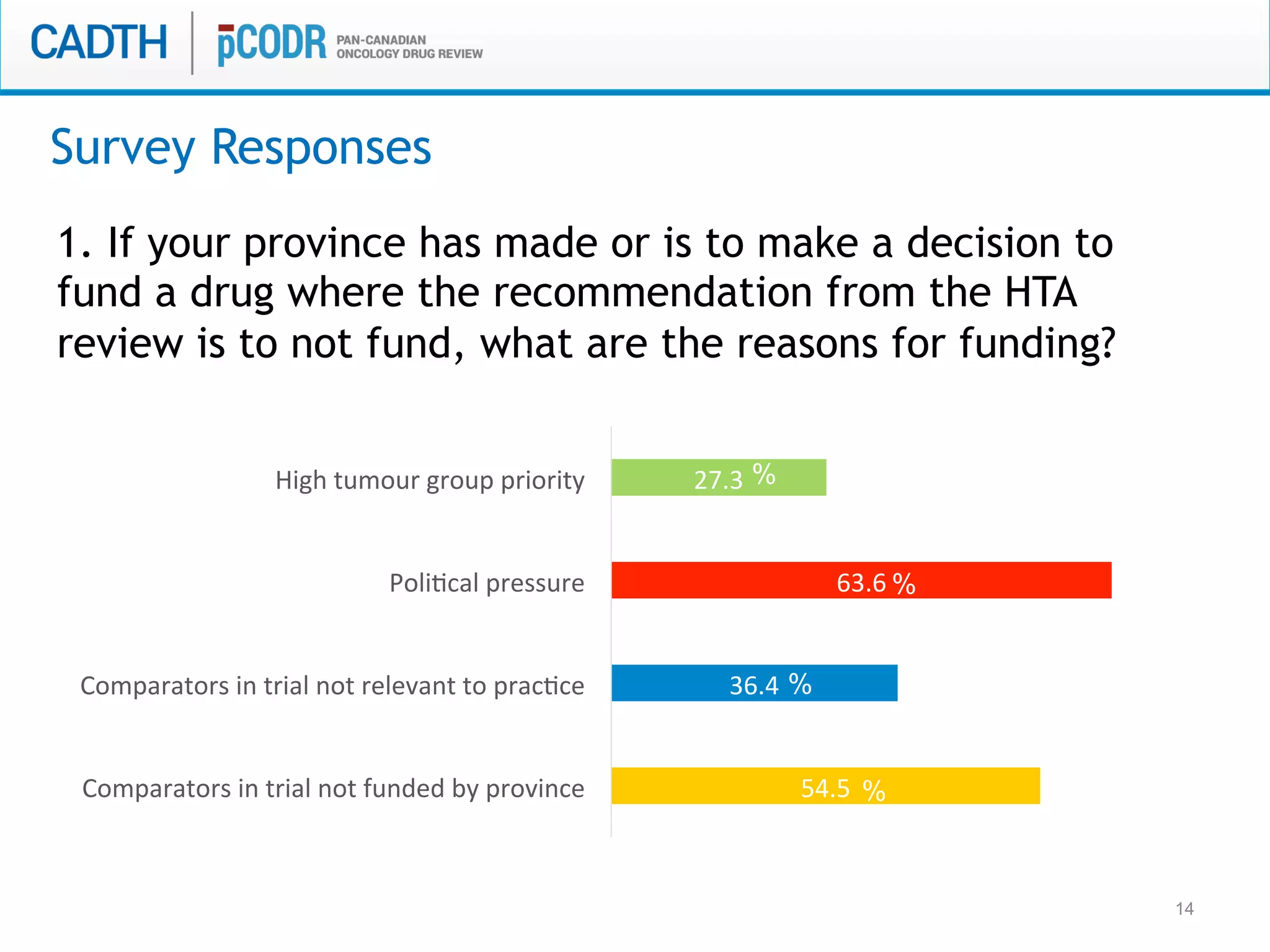
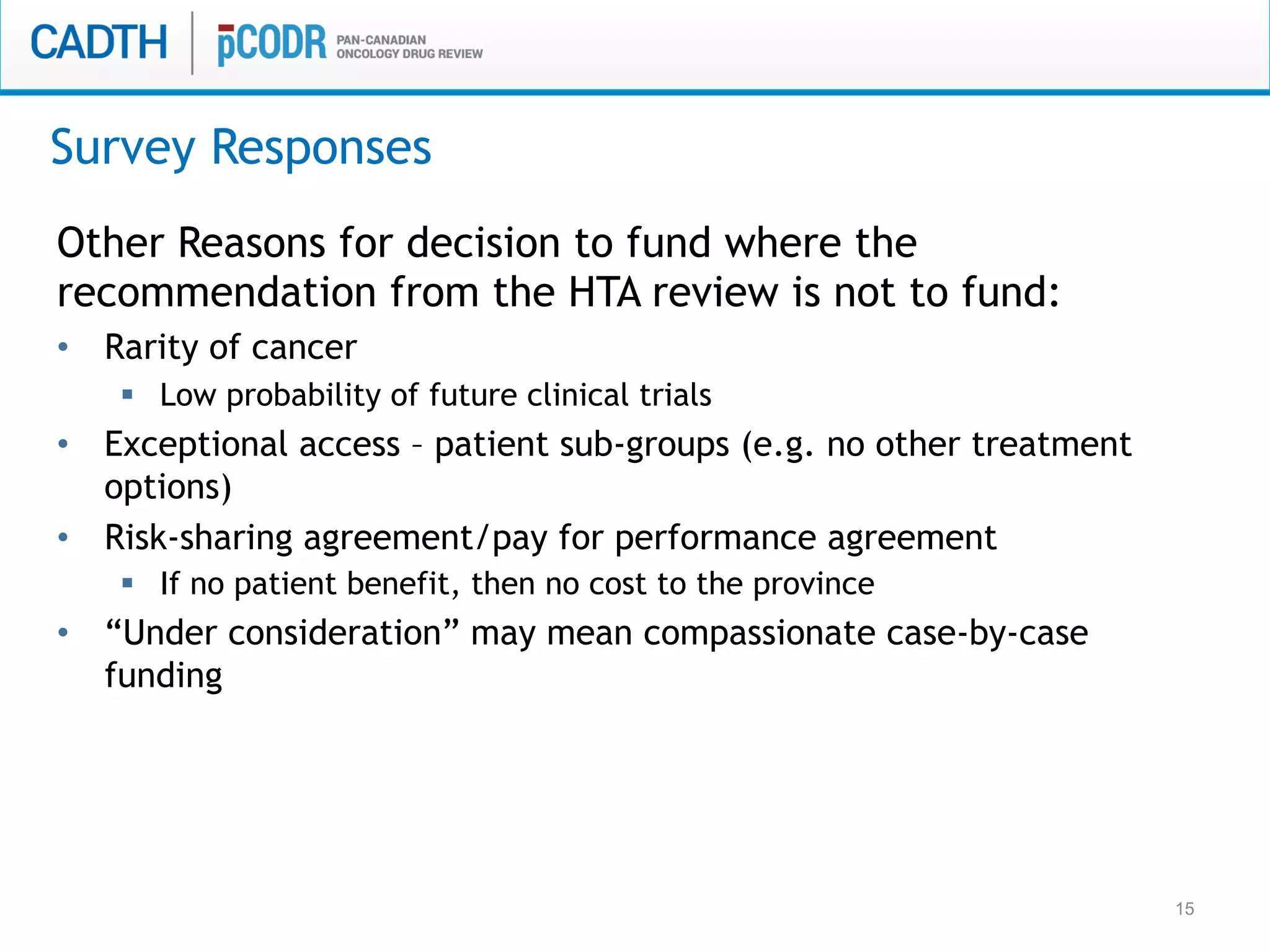
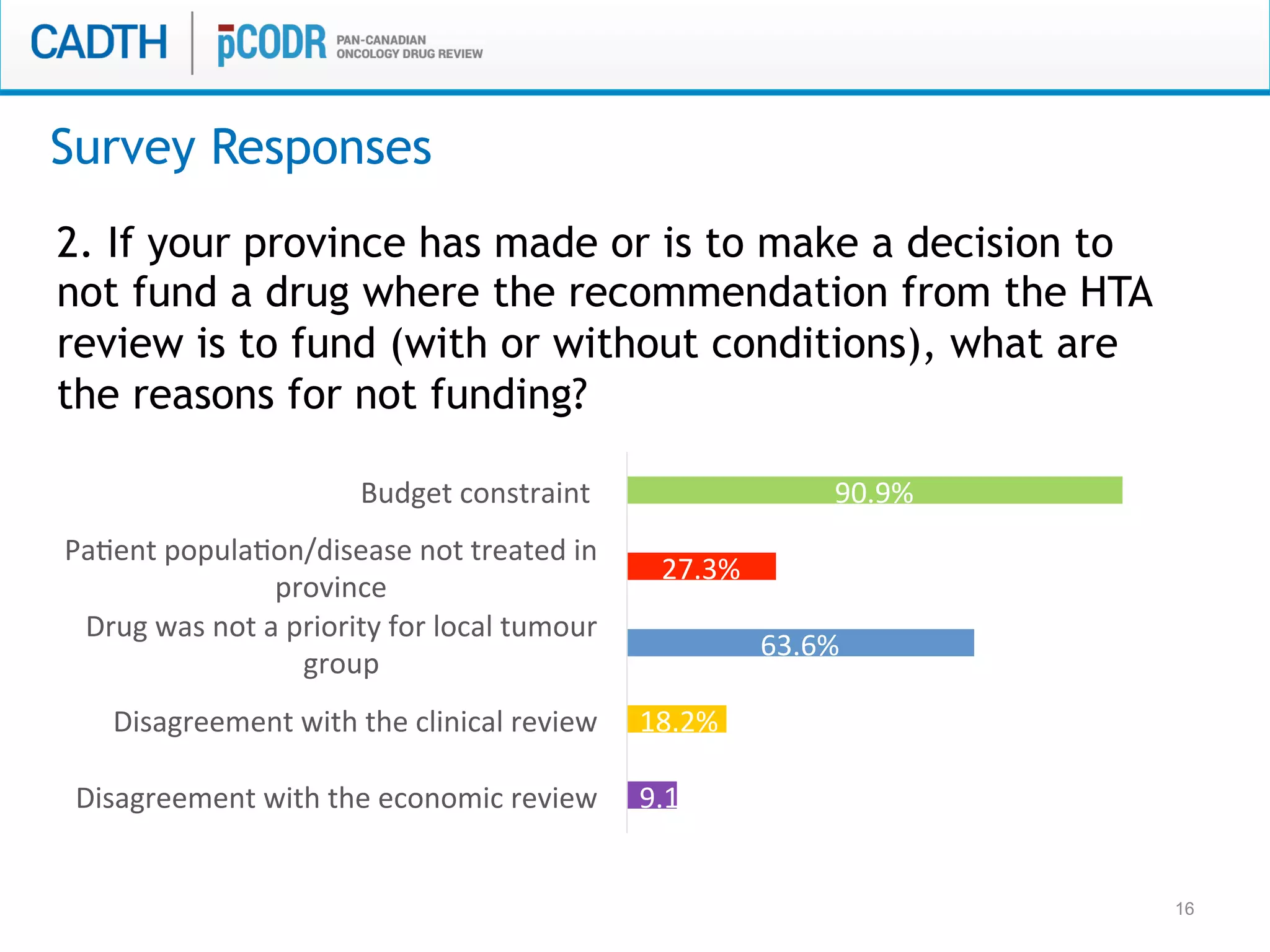
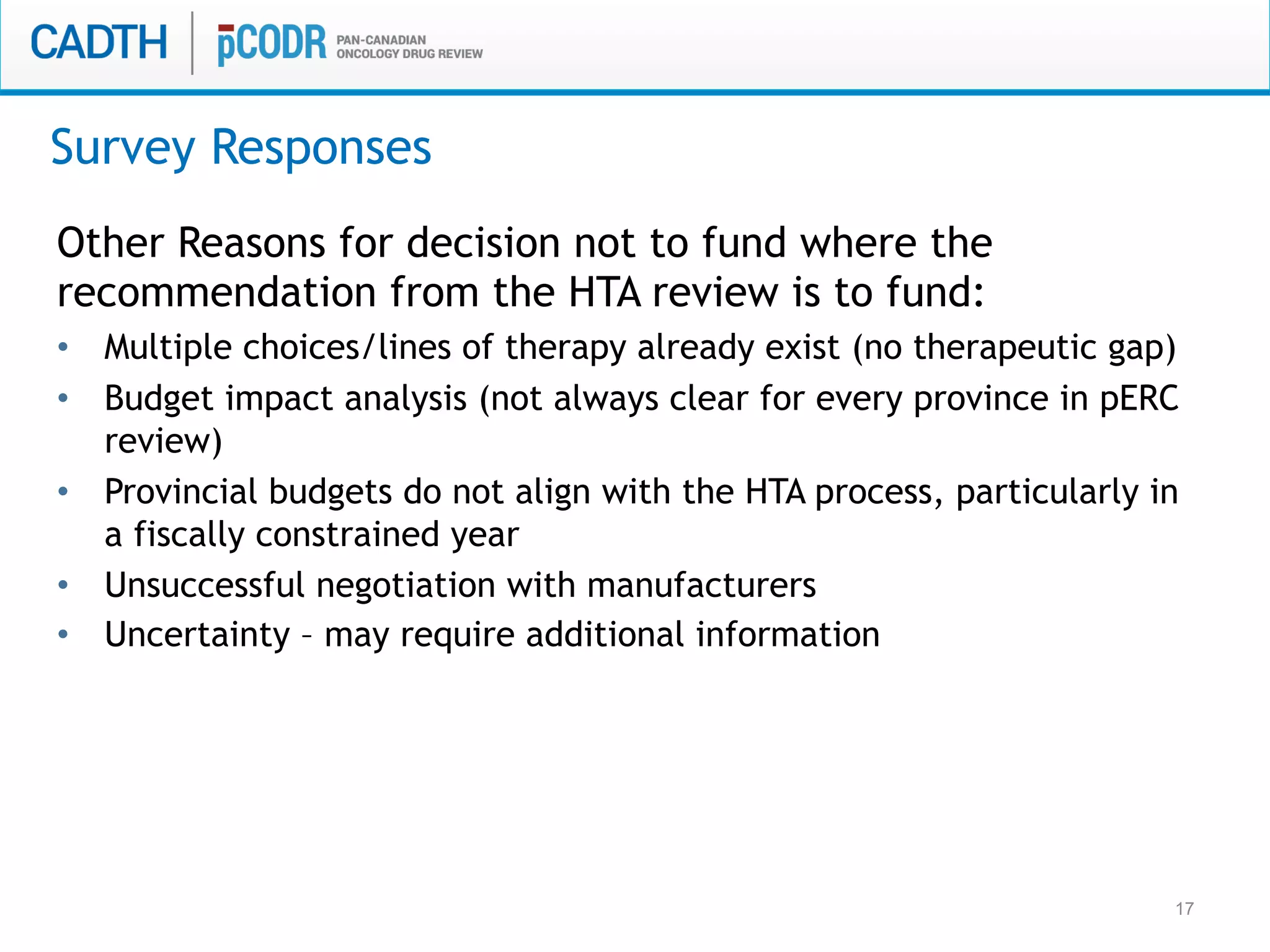
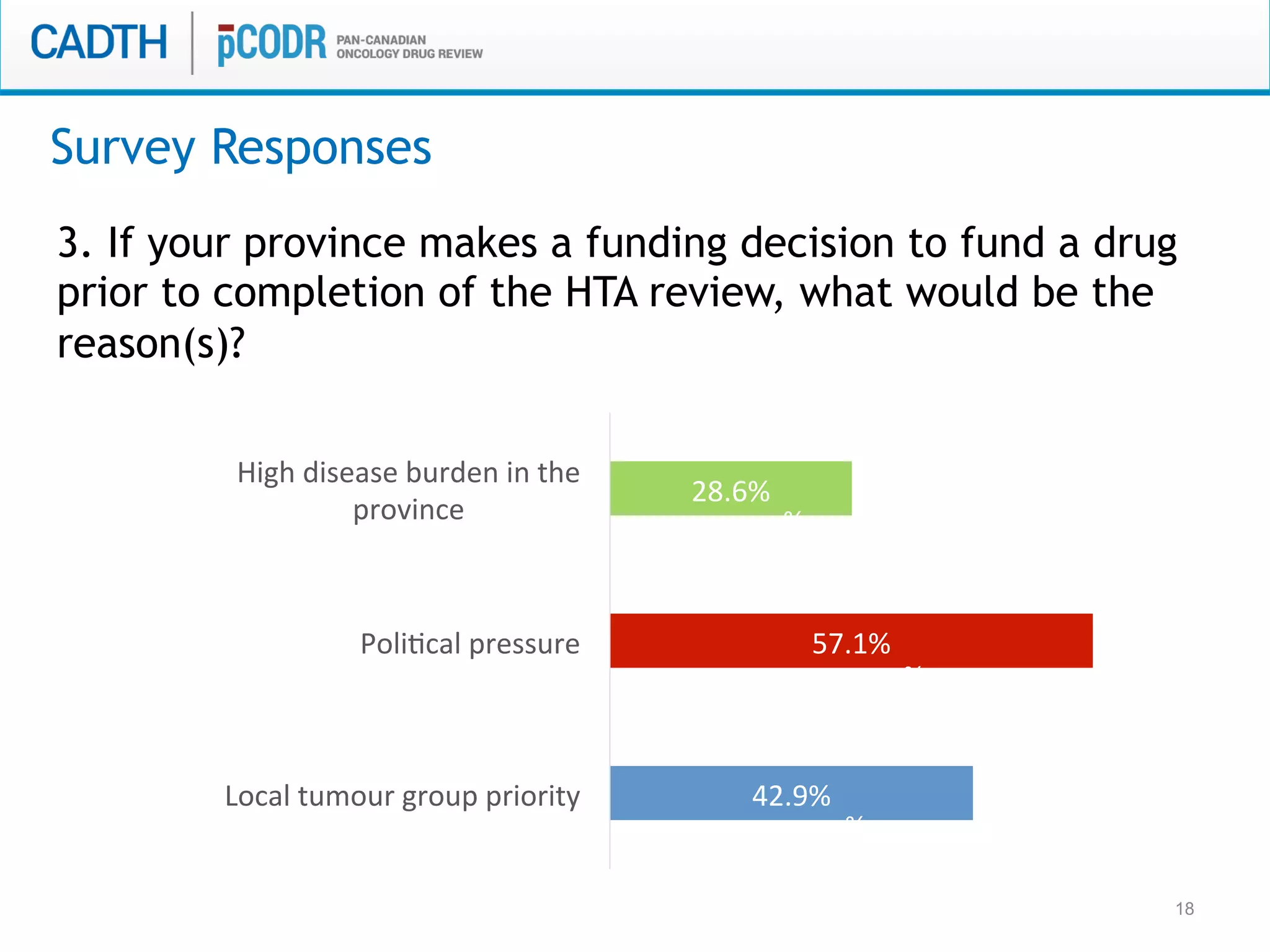
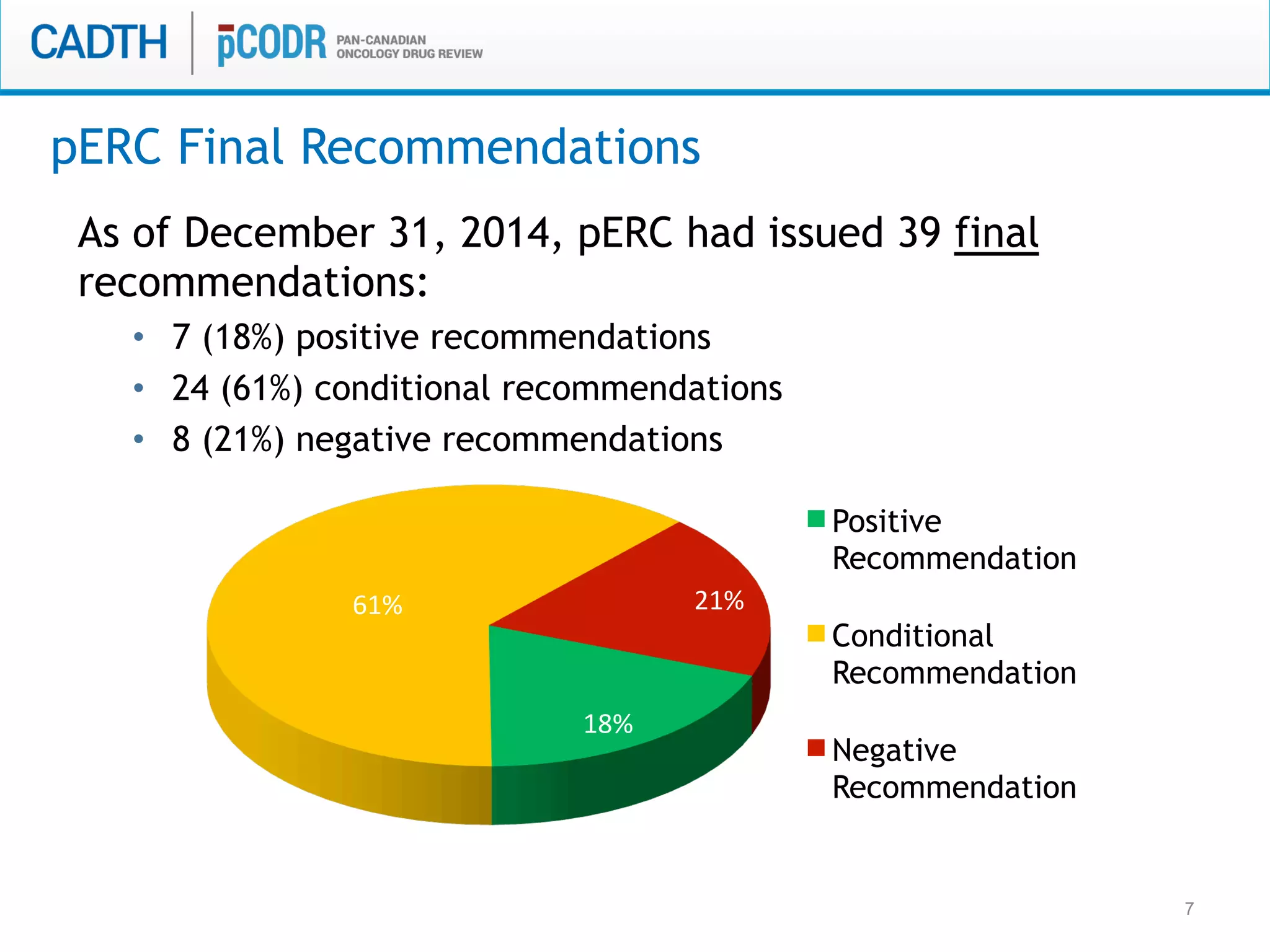
The document discusses discordance between recommendations from the pan-Canadian Oncology Drug Review (pERC) and provincial funding decisions for cancer drugs in Canada. It presents results from a survey of provincial drug funding decision-makers that identified several potential reasons for discordance, including differences in priorities, clinical evidence, and budget constraints between the levels. Challenges to alignment included issues with clinical trial evidence as well as pressure to expand access, while suggested solutions centered on strengthening consensus around treatment pathways and priorities nationally.






![9
pERC Recommendations to Fund
1. Pazopanib (Votrient) for
metastatic renal cell
carcinoma [Jan 20, 2012]
2. Bendamustine hydrochloride
(Treanda) for NHL [Dec 14,
2012]
3. Axitinib (Inlyta) for
metastatic renal cell
carcinoma [Apr 11, 2013]
4. Bortezomib (Velcade) for
multiple myeloma, pre-
ASCT [Apr 11, 2013]
5. Pazopanib (Votrient)
resubmissions for
metastatic renal cell
carcinoma [Sep 16, 2013]
6. Afatinib (Giotrif) for non-
small cell lung cancer [May
20, 2014]
7. Arsenic trioxide (Trisenox)
for Acute Promyelocytic
Leukemia [Mar 5, 2014]
0%
10%
20%
30%
40%
50%
60%
70%
80%
90%
100%
1 2 3 4 5 6 7
Provincial Funding as of December 31, 2014
Funded Under Negotiation with Manufacturer
Under Provincial Consideration Not Funded](https://image.slidesharecdn.com/cadth2015b1slidesallangrill-pcodr-cadthsymposium2015presentationfinal-150414101544-conversion-gate01/75/Cadth-2015-b1-slides-allan-grill-pcodr-cadth_symposium2015presentationfinal-9-2048.jpg)
![10
pERC Recommendations to Not Fund
1. Pazopanib (Votrient) for soft
tissue sarcoma [Dec 14,
2012]
2. Bendamustine hydrochloride
(Treanda) for CLL [Dec 14,
2012]
3. Bortezomib (Velcade) for
multiple myeloma, post-
ASCT [Apr 11, 2013]
4. Lapatinib (Tykerb) for
breast cancer [Jul 22, 2013
5. Regorafenib (stivarga) for
metastatic colorectal
cancer [Dec 2, 2013]
6. Cetuximab (Erbitux0 for
metastatic colorectal
cancer [Jan 27, 2014]
7. Aflibercept (Zaltrap) for
metastatic colorectal
cancer [Sep 22, 2014]
0%
10%
20%
30%
40%
50%
60%
70%
80%
90%
100%
1 2 3 4 5 6 7
Provincial Funding as of December 31, 2014
Funded Under Negotiation with Manufacturer
Under Provincial Consideration Not Funded](https://image.slidesharecdn.com/cadth2015b1slidesallangrill-pcodr-cadthsymposium2015presentationfinal-150414101544-conversion-gate01/75/Cadth-2015-b1-slides-allan-grill-pcodr-cadth_symposium2015presentationfinal-10-2048.jpg)