This document discusses adaptive clinical trial designs used by Merck for oncology studies. It provides examples of different types of adaptive designs including:
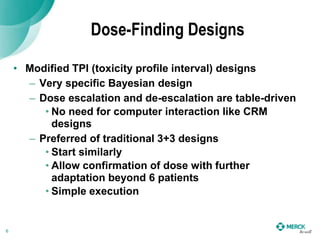
1) Dose-finding designs like modified toxicity profile interval designs that allow for dose escalation and de-escalation in a table-driven manner.
2) Single-arm response rate studies that can provide early access for patients and allow early interim analyses to discontinue biomarker-negative patient populations if no responses are found.
3) Comparative studies with dual primary endpoints of overall survival and progression-free survival that can adapt based on biomarker-selected patient populations at interim analyses.
4) Bayesian adaptive studies like I-SPY 2 that