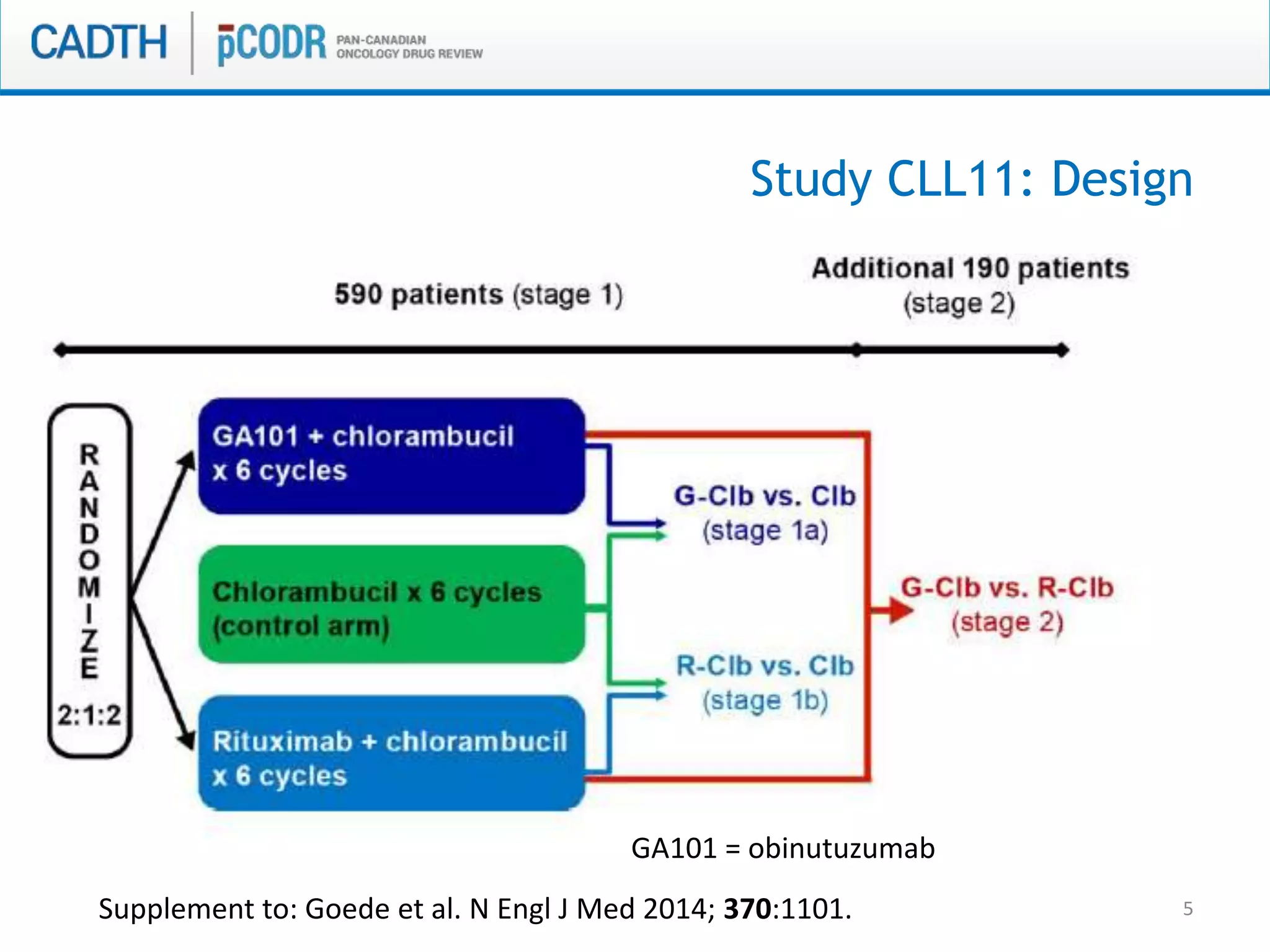
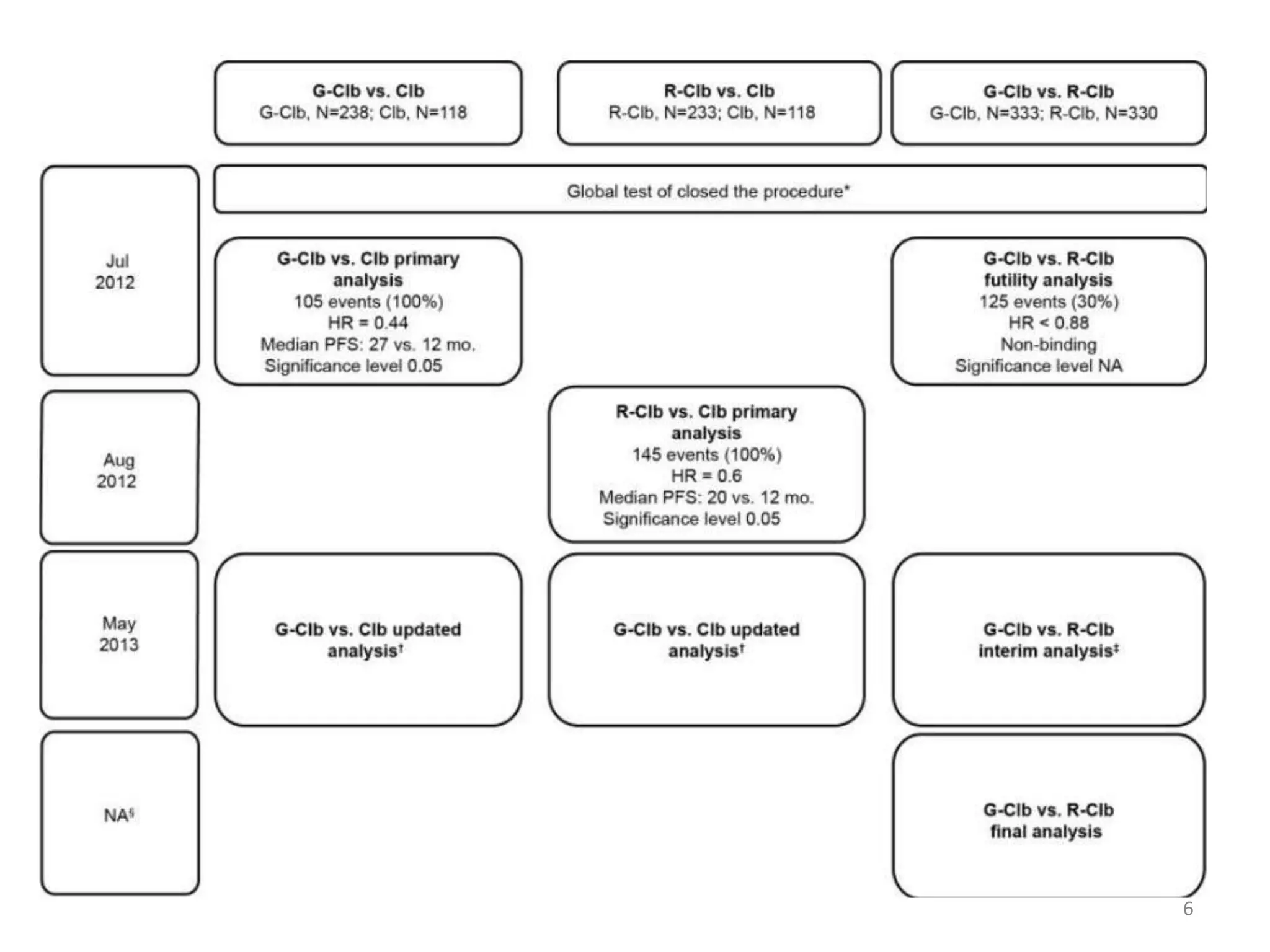
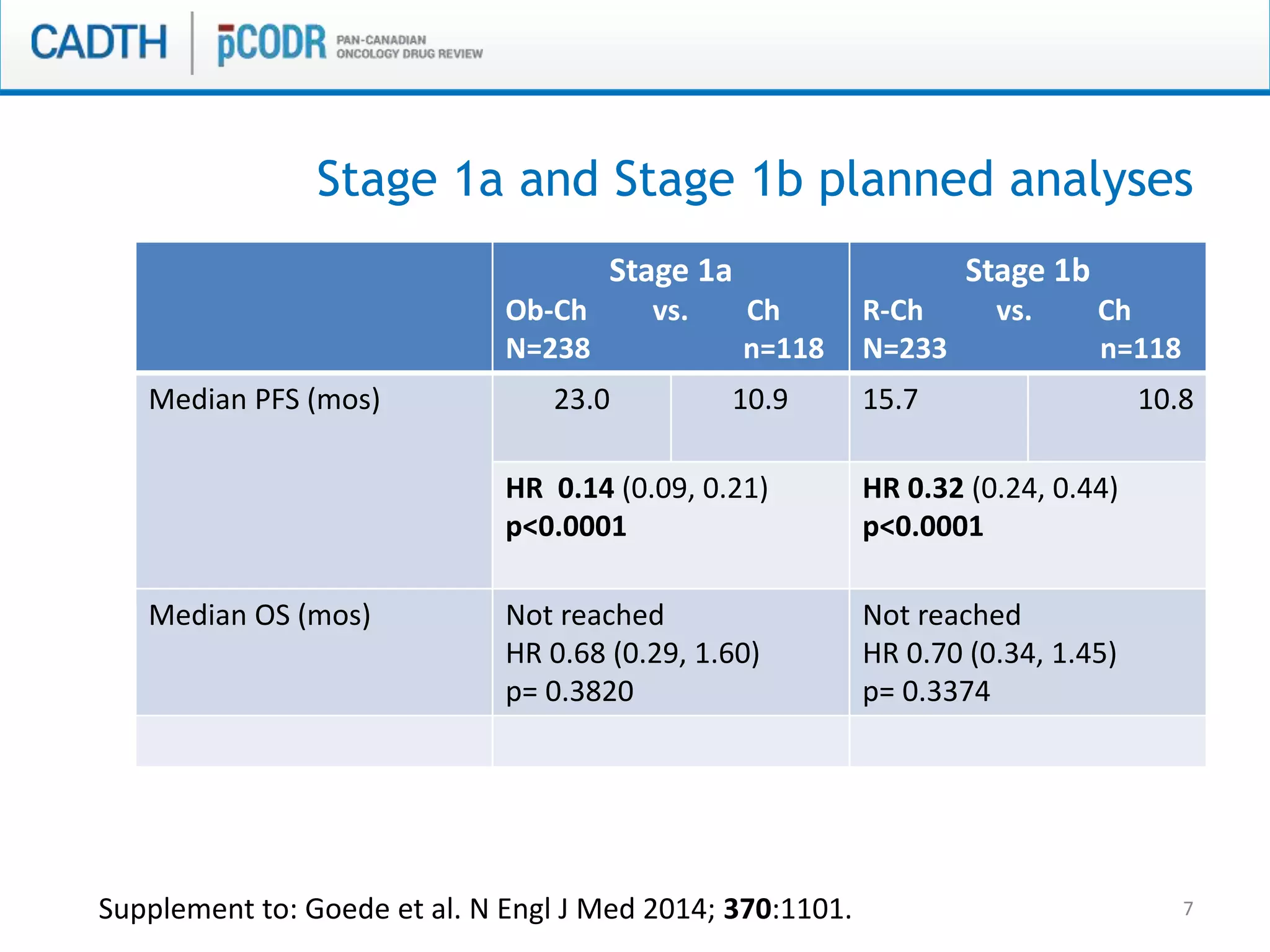
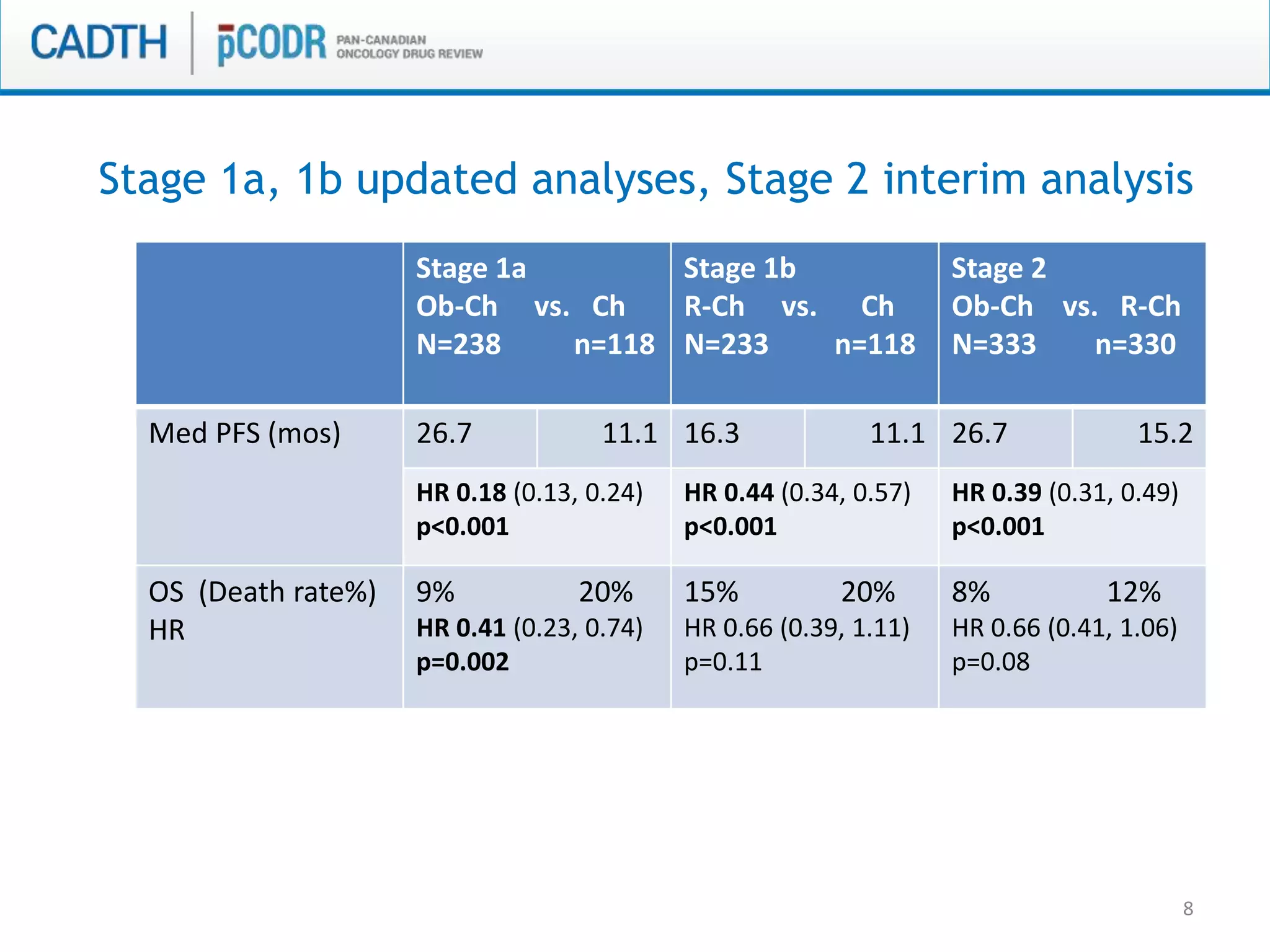
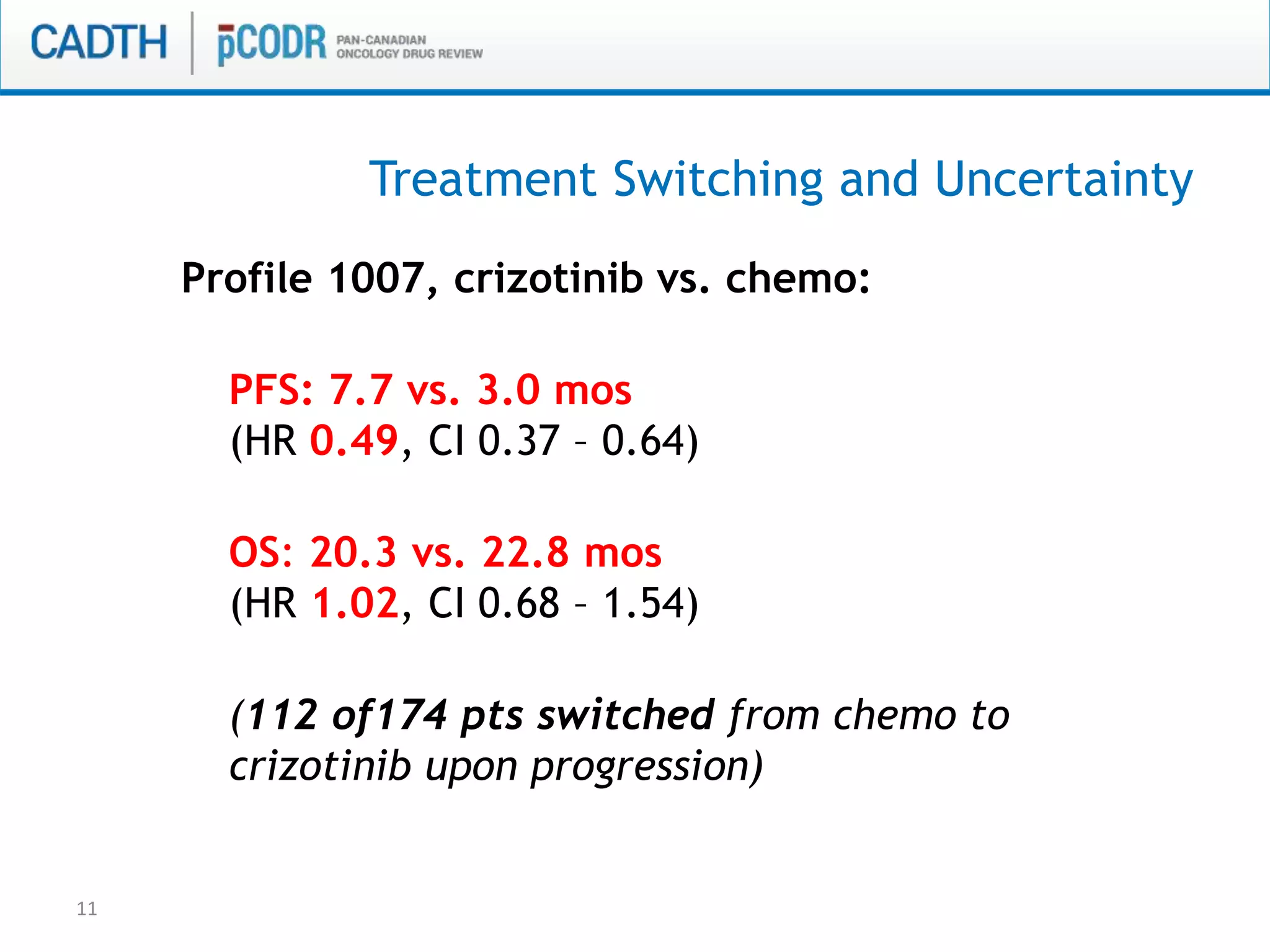
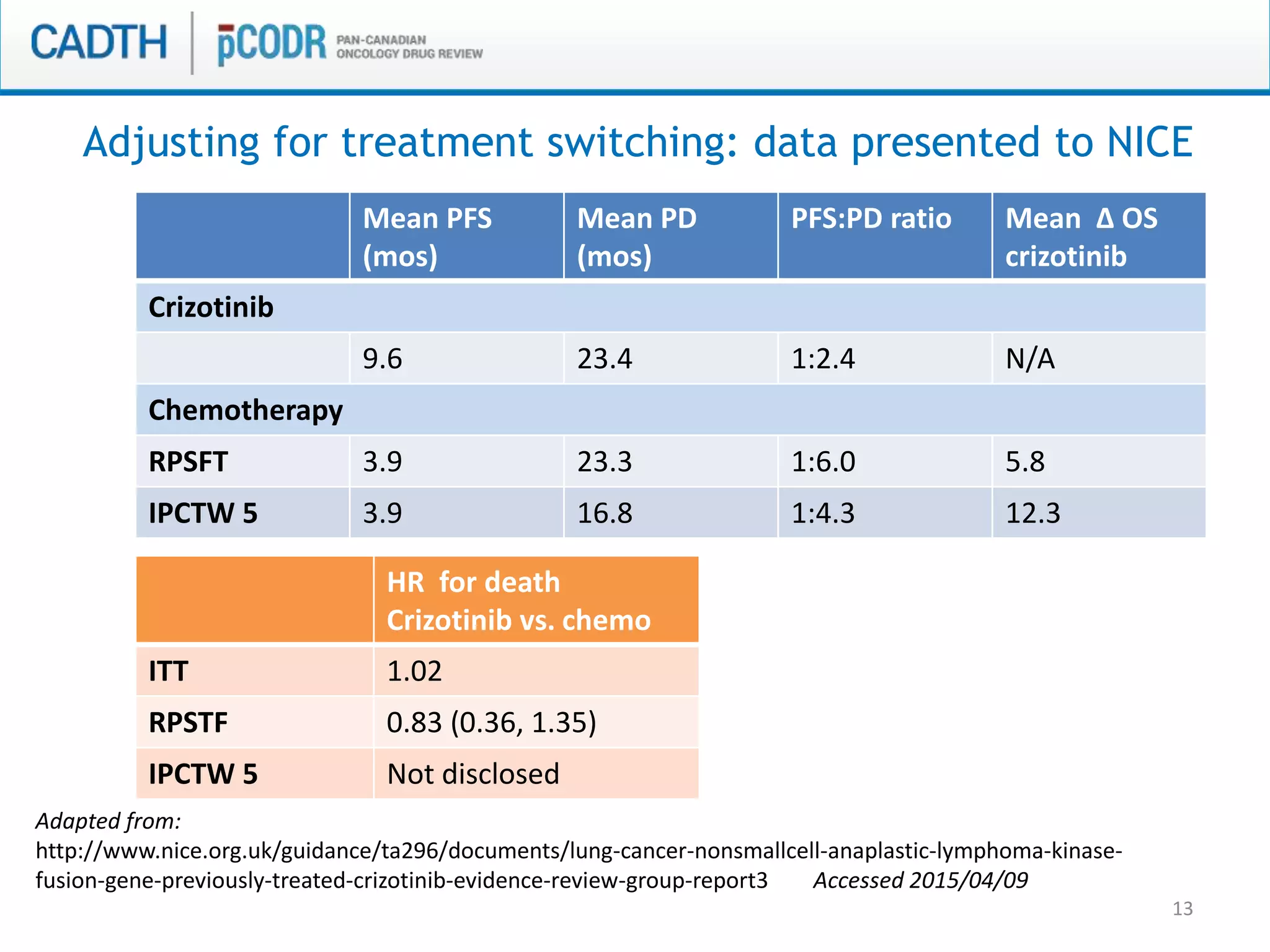
This document discusses pCODR's experience with adaptive clinical trial designs and how they impact drug funding assessments. It provides examples of two clinical trials for chronic lymphocytic leukemia (CLL) and non-small cell lung cancer (NSCLC) that used adaptive designs. The CLL trial had early stopping rules and treatment switching based on interim analyses. The NSCLC trial allowed patients who progressed on chemotherapy to switch to the experimental drug crizotinib, introducing uncertainty into estimating the treatment's effectiveness and cost-effectiveness. pCODR will likely see more complex adaptive designs as cancer treatments increasingly target biomarker-defined subgroups and employ personalized medicine approaches, requiring continuous evolution of its assessment expertise.