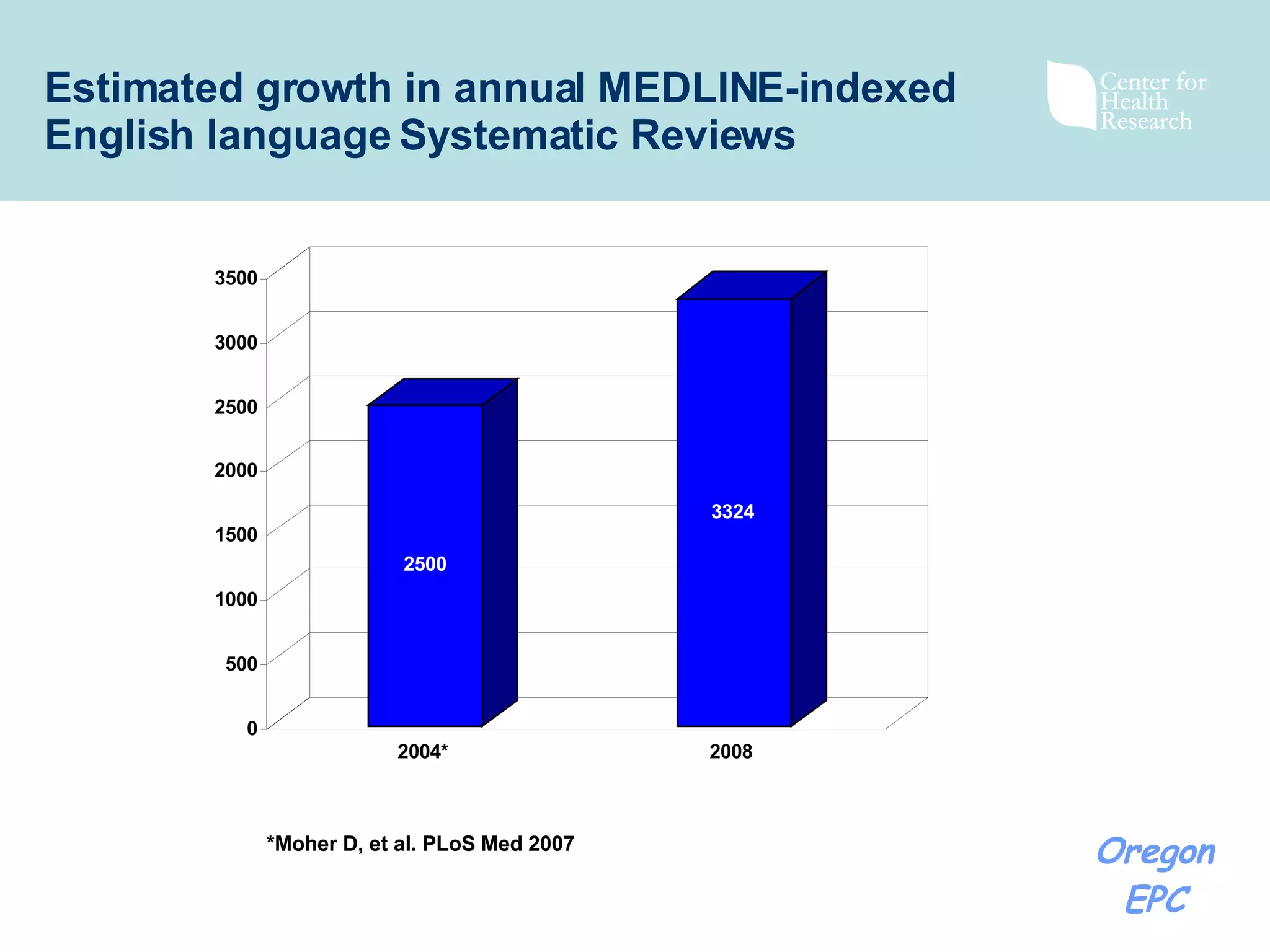
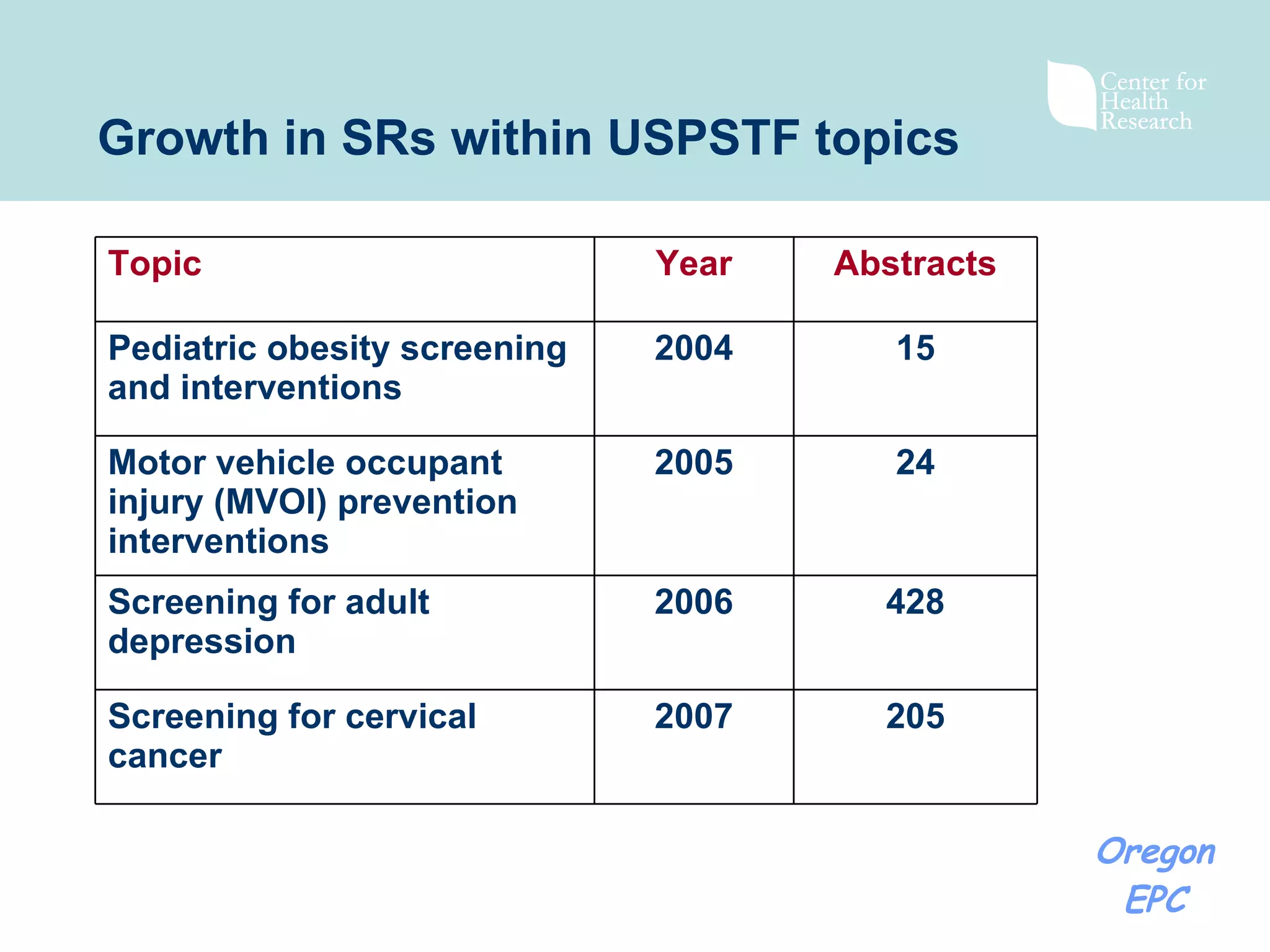
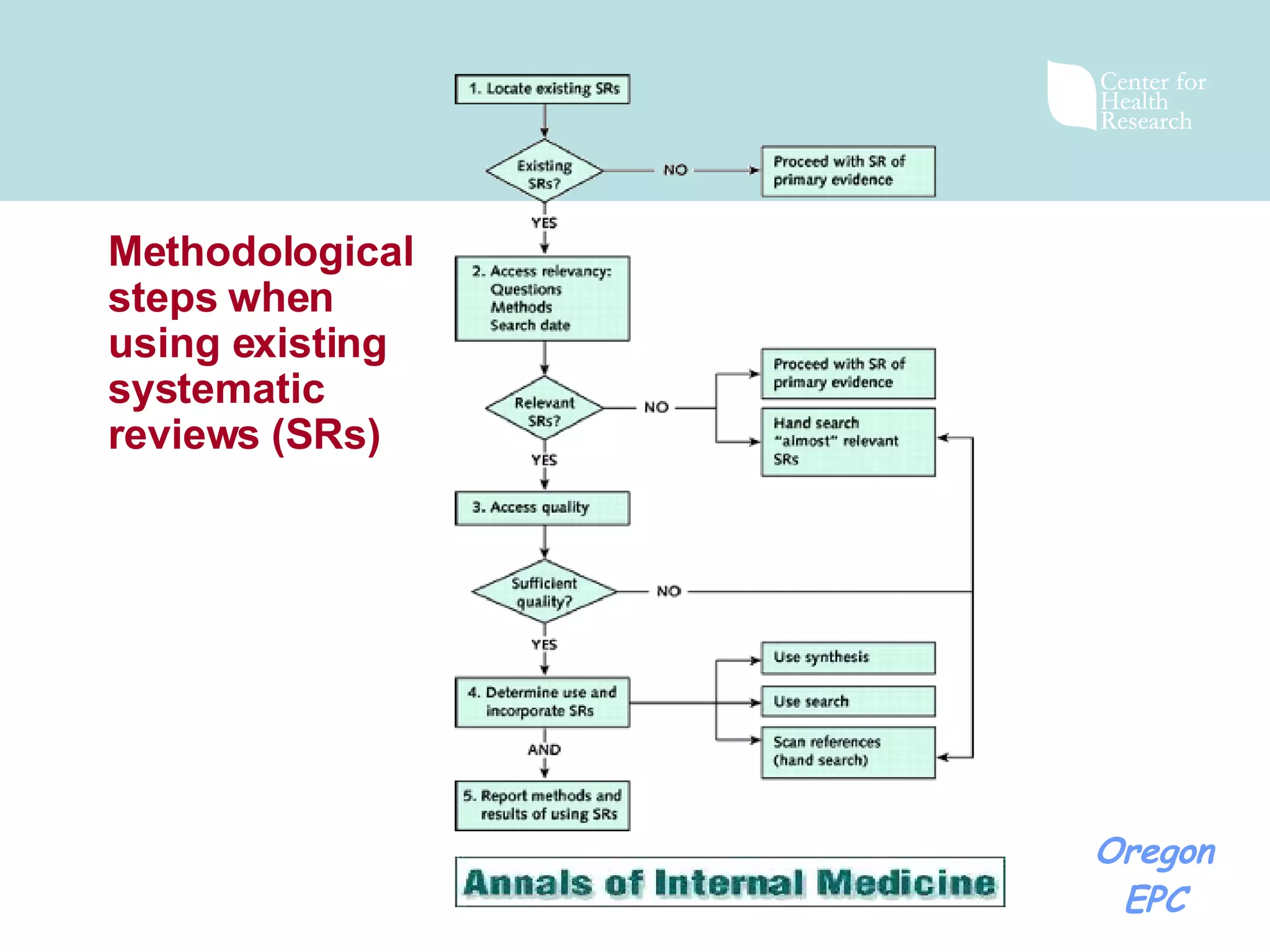
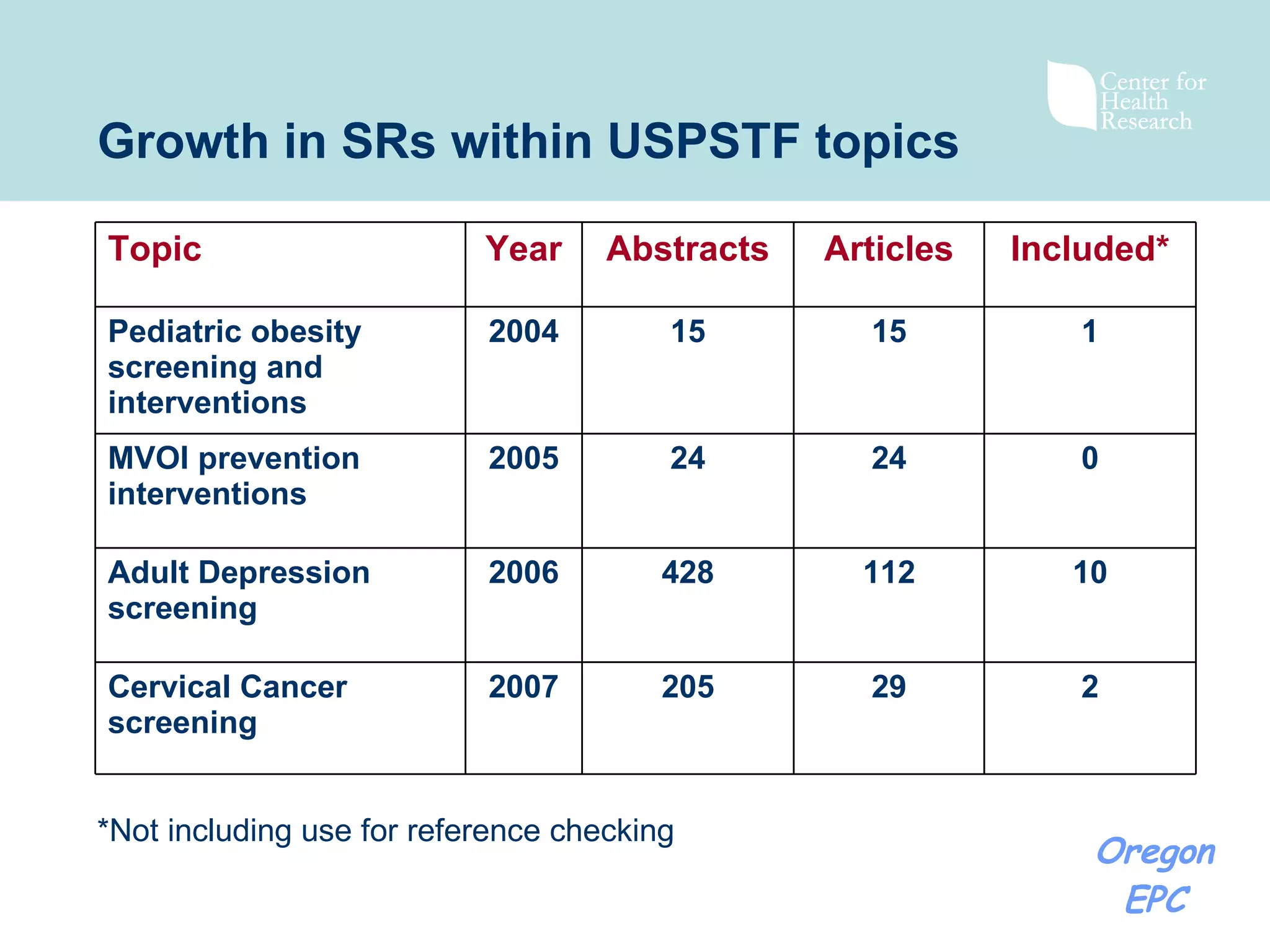
There has been exponential growth in the number of systematic reviews published each year, creating challenges for reviewers seeking to use existing reviews in new reviews. While using existing reviews could save time and resources, current review methods do not provide clear guidance on how to appropriately incorporate existing reviews. Key issues include determining whether existing reviews are valid and applicable to the new review's questions, and whether a new review is still needed to address limitations of prior reviews. Standardizing review reporting and developing formal processes for review updating or replacement could help address these challenges and reduce unnecessary duplication of effort across reviews.