Embed presentation
Download as PDF, PPTX



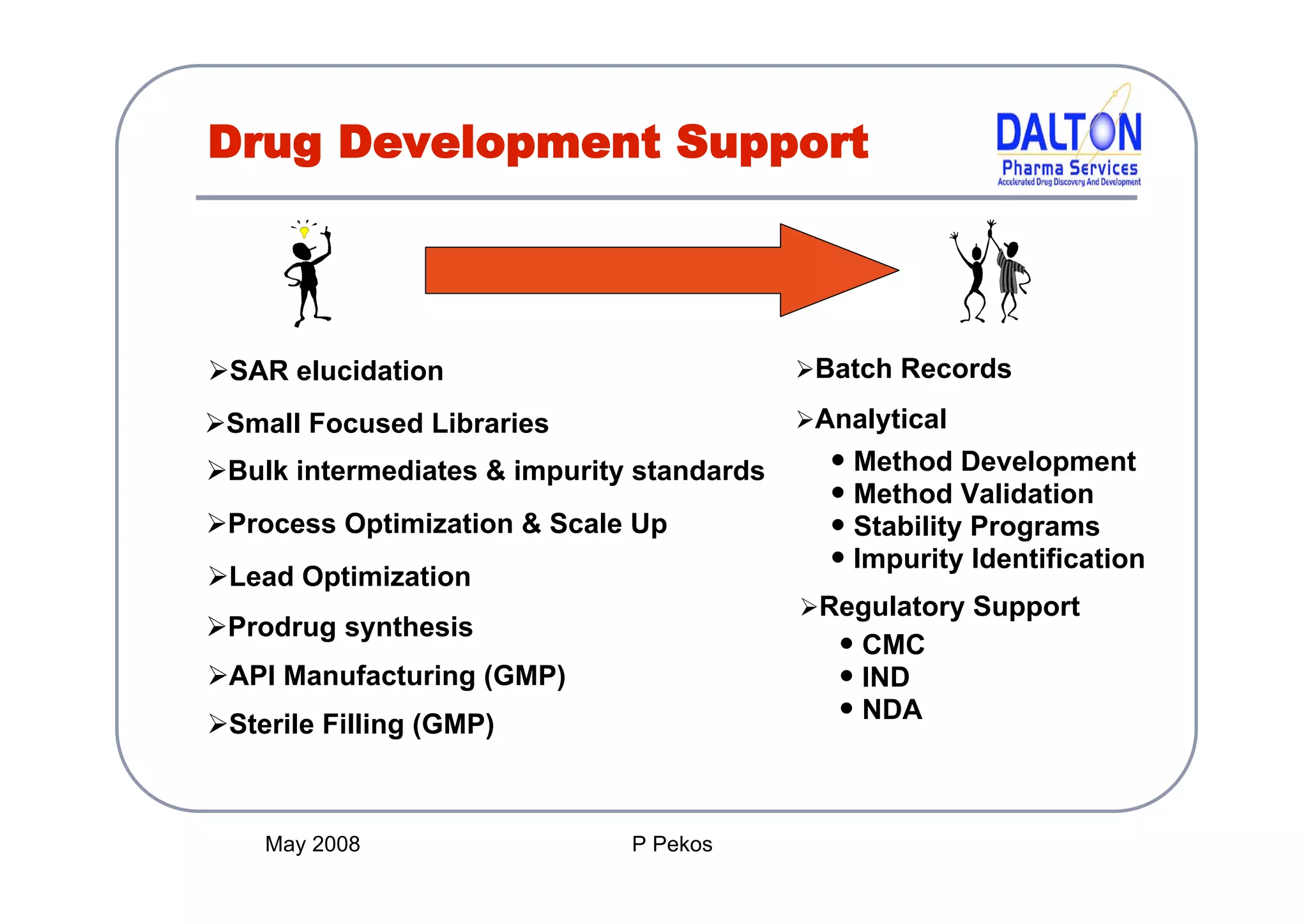



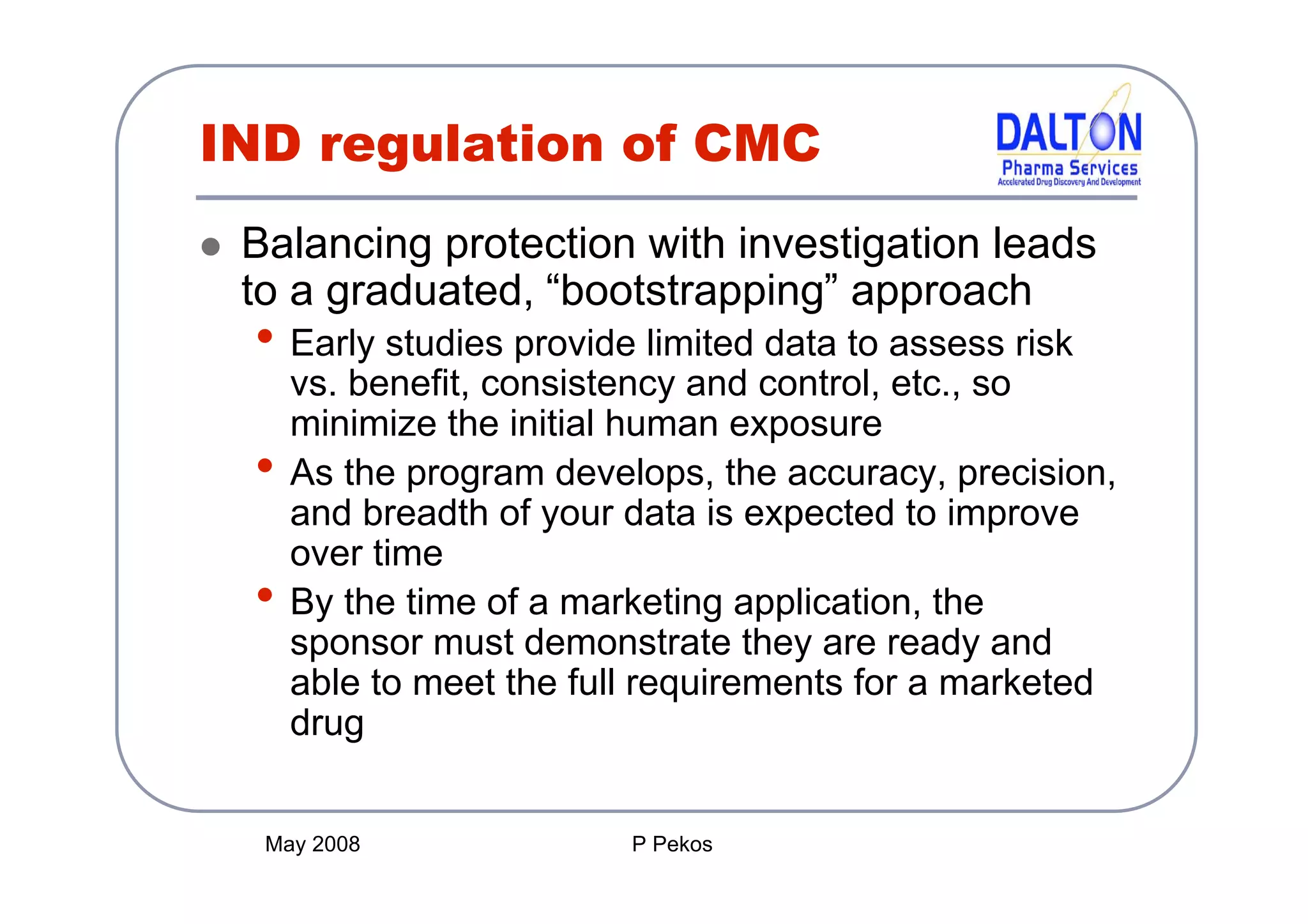
















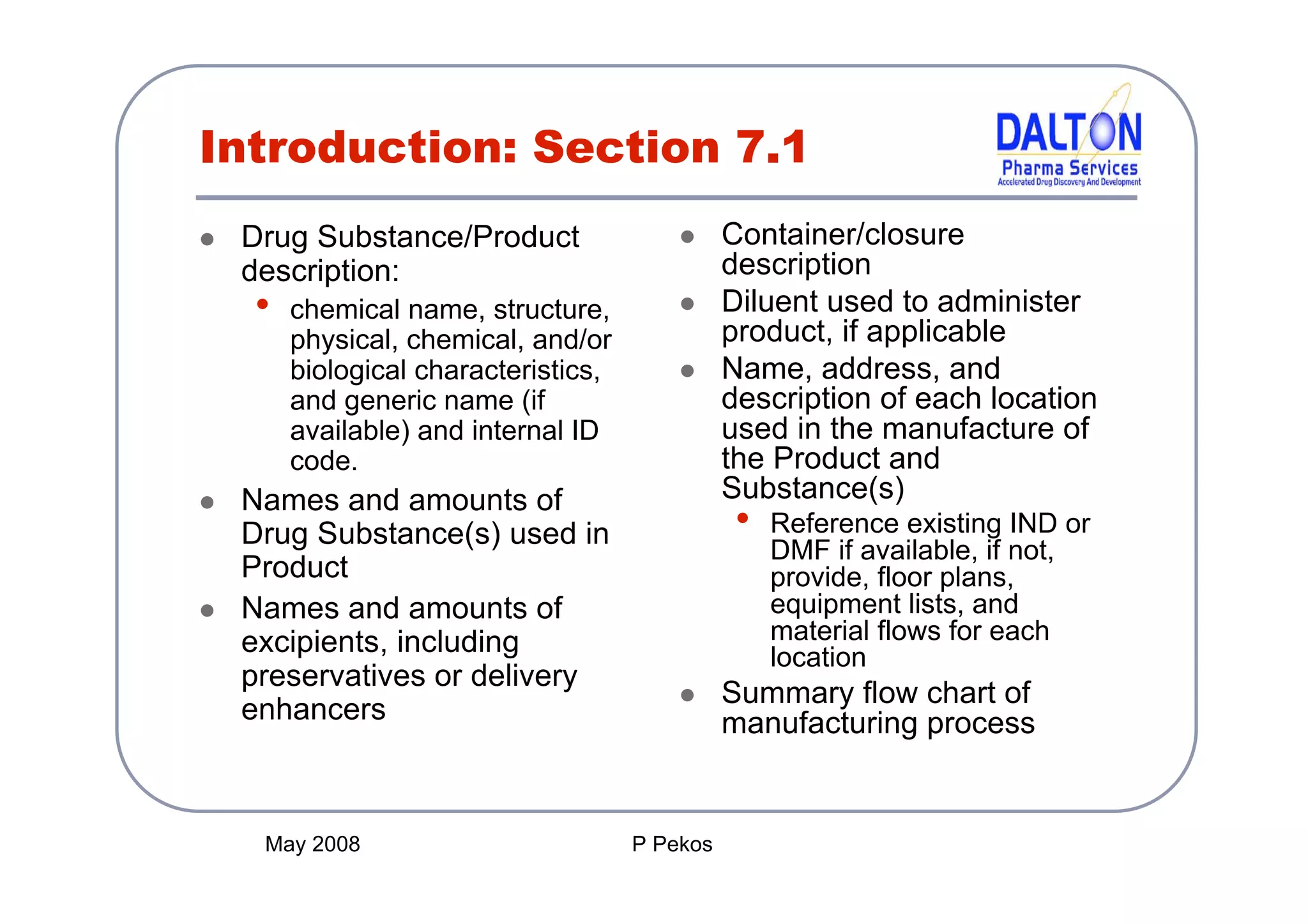











The document outlines the principles and content requirements for preparing an Investigational New Drug (IND) application, emphasizing the complexity of drug development and the regulatory frameworks that govern it. It details the significance of Chemistry, Manufacturing, and Controls (CMC) including good manufacturing practices (cGMP), key requirements for drug substance and product characterization, and potential causes for clinical holds. Additionally, it provides resources and considerations for compliance with regulatory expectations throughout the drug development process.


































