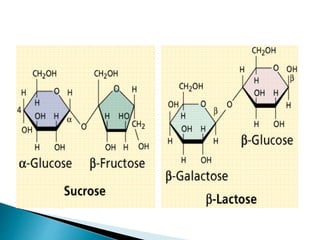
Carbohydrates originate from photosynthesis in plants. They serve several functions in plants and animals including energy storage, structure, and participating in biochemical processes. Carbohydrates are classified based on their size as monosaccharides, oligosaccharides, or polysaccharides. Monosaccharides are the simplest form, oligosaccharides contain 2-10 monosaccharide units, and polysaccharides are long chains of many monosaccharide units. Common examples of each type are also provided.