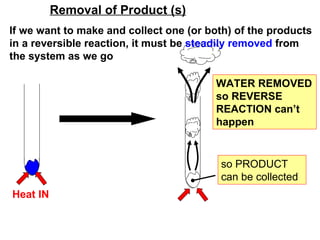
The document discusses reversible chemical reactions and the Haber process for producing ammonia from nitrogen and hydrogen. The Haber process involves a reversible reaction where nitrogen and hydrogen gases react to form ammonia gas, which can also break down into the original reactants. To maximize ammonia production, the reaction is run at high pressure and a temperature that balances a reasonable yield with a fast reaction rate. Ammonia is removed from the system to drive the reaction towards producing more product rather than reversing.