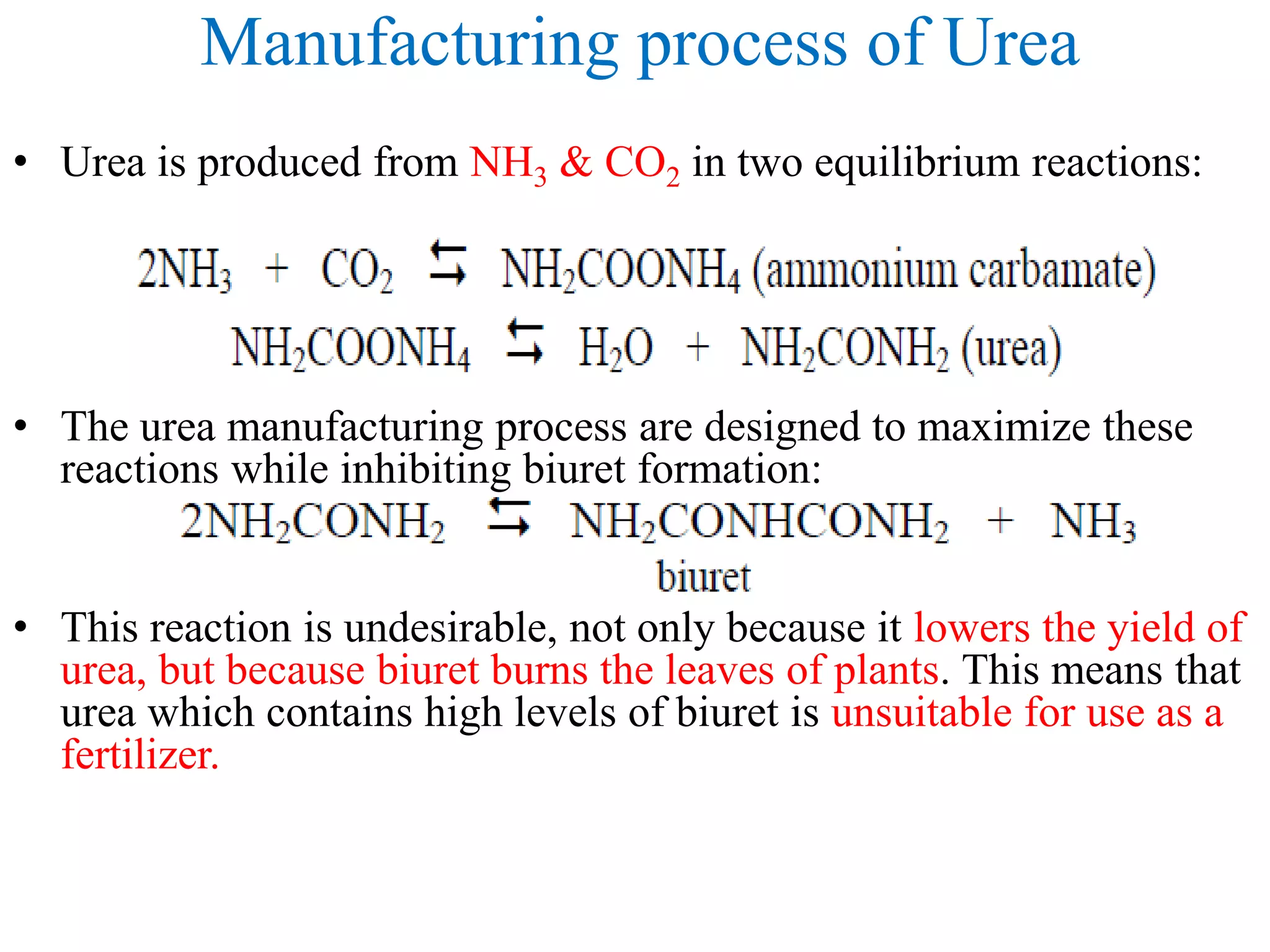
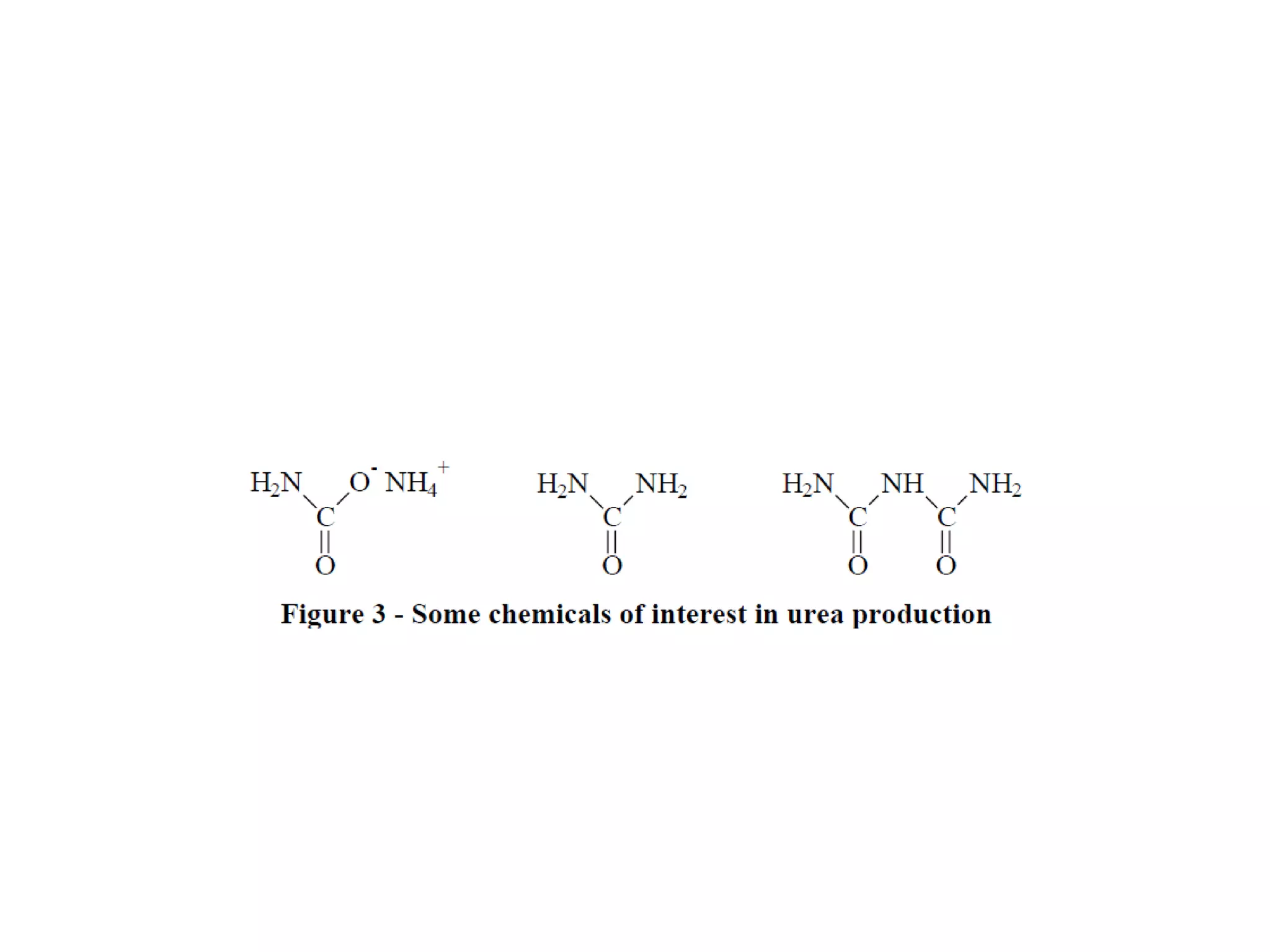
1. Ammonia is produced through the Haber process where nitrogen and hydrogen react over an iron catalyst at high temperatures and pressures.
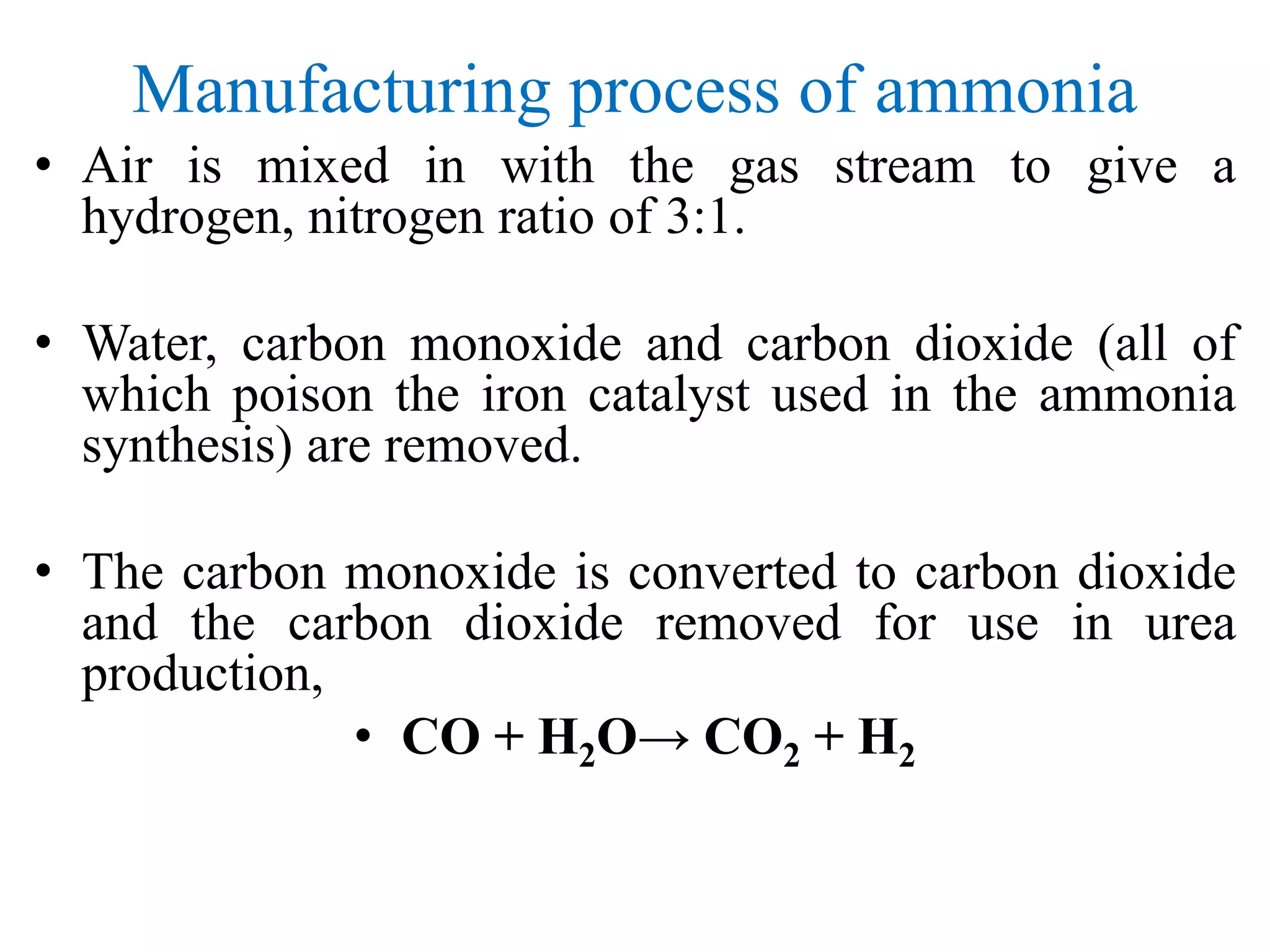
2. Hydrogen is produced from natural gas through steam reforming, and nitrogen is obtained from air.
3. The synthesis gas undergoes several purification steps including desulfurization, shift conversion and CO2 removal before being compressed and fed into the ammonia reactor.
4. In the ammonia reactor, only 10-20% of the gases react to form ammonia, with the unreacted gases recycled and fresh gases added to maintain equilibrium.
































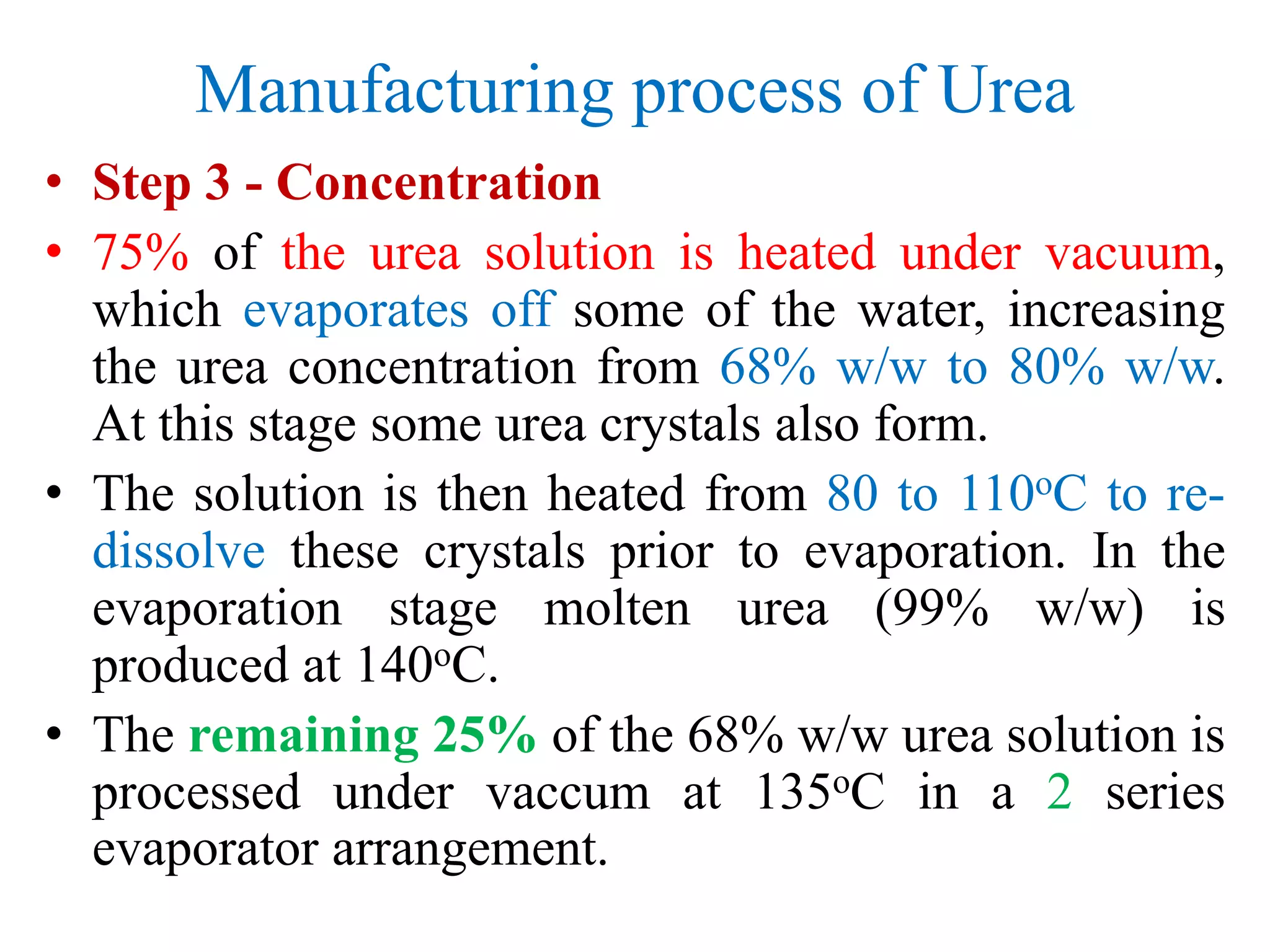


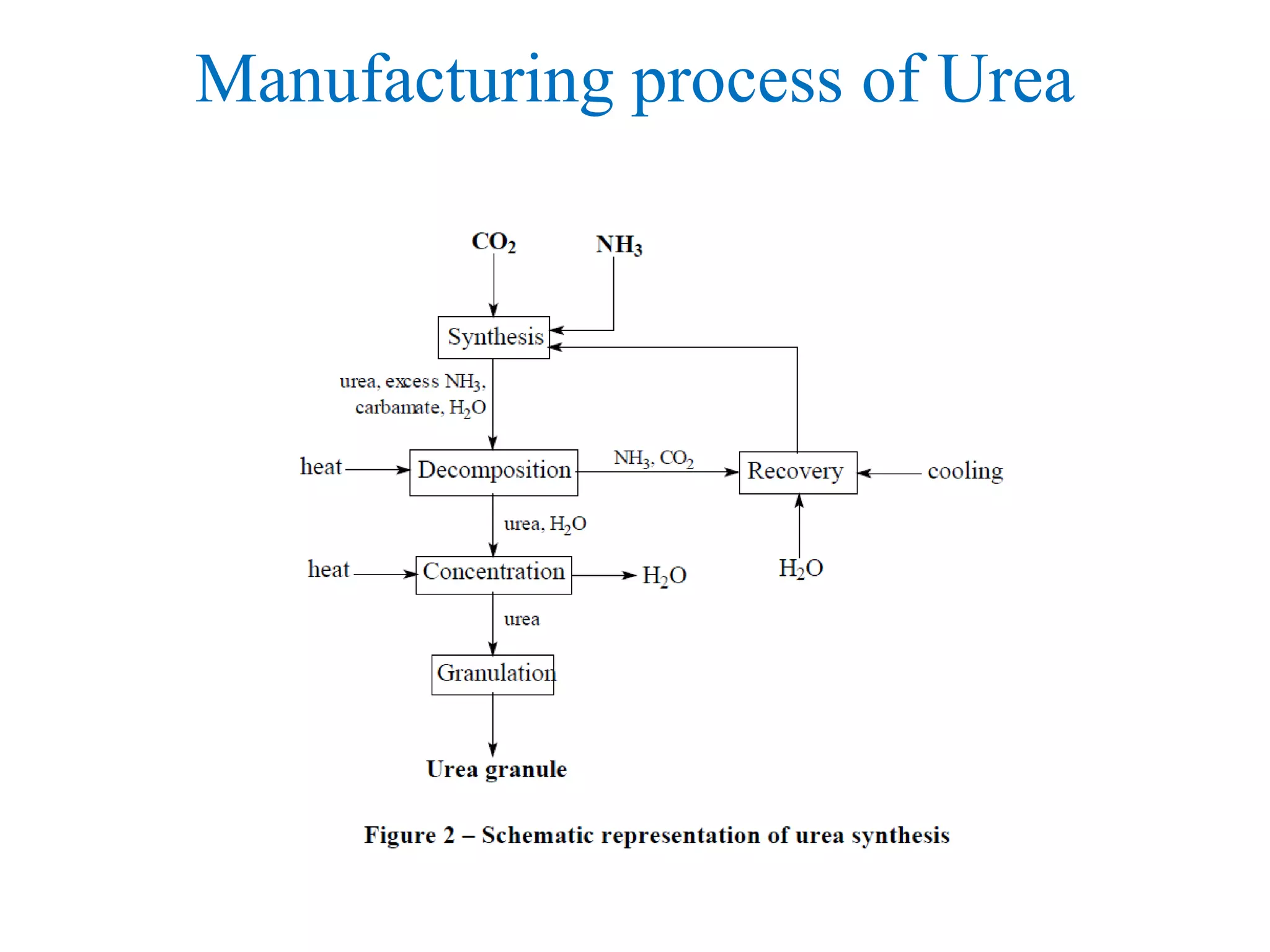


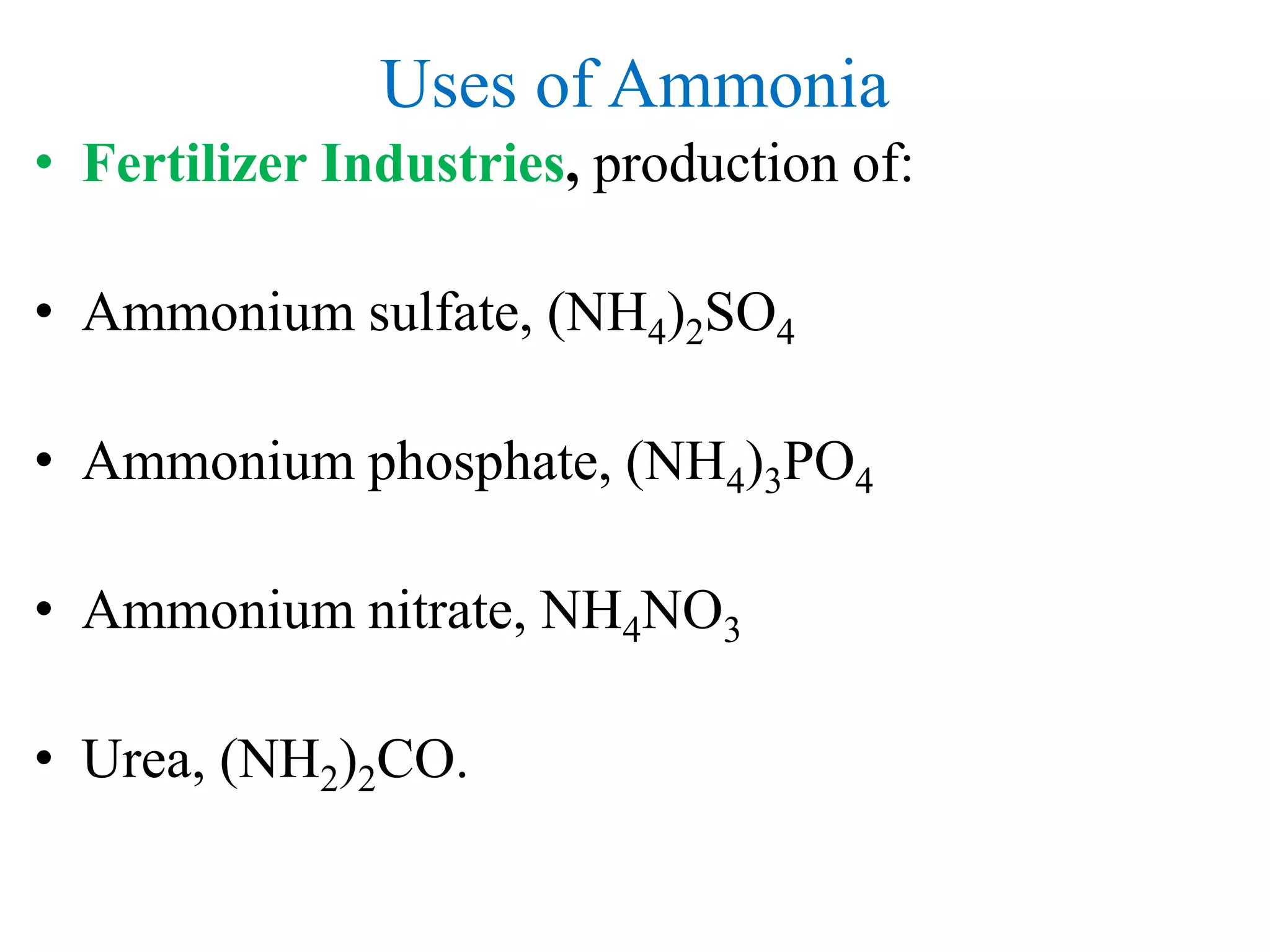

![Uses of Ammonia
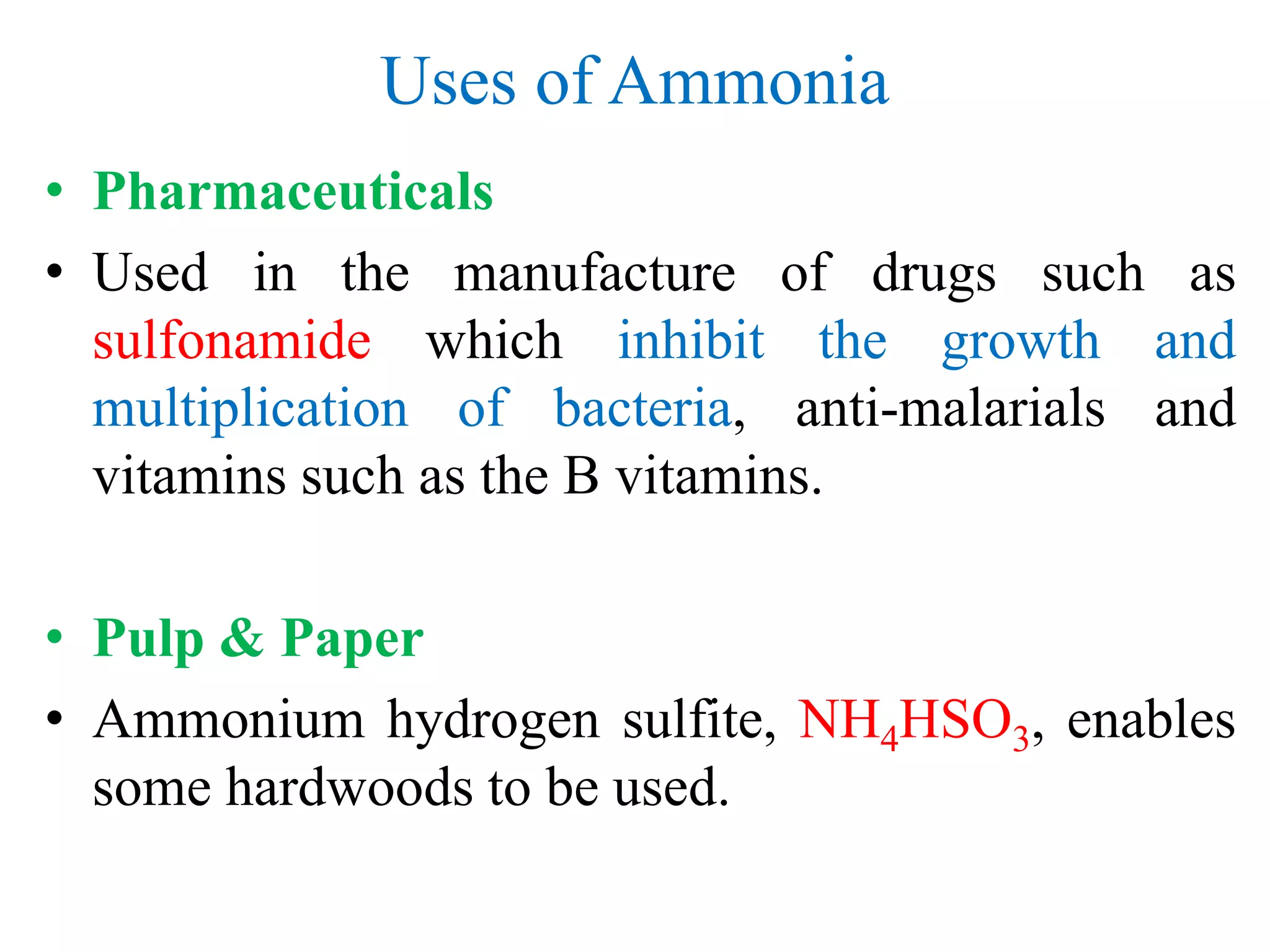
• Explosives
• Ammonium nitrate, NH4NO3
• Fibres & Plastic
• Nylon, -[(CH2)4-CO-NH-(CH2)6-NH-CO]-,and other
polyamides.
• Refrigeration
• Used for making ice, large scale refrigeration plants,
air-conditioning units in buildings and plants](https://image.slidesharecdn.com/ammoniaurea-180121221930/75/Ammonia-and-urea-production-44-2048.jpg)