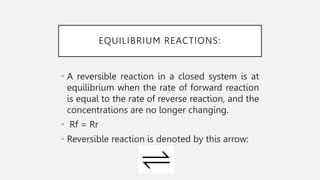
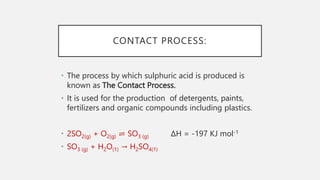
The document discusses reversible reactions and equilibrium reactions. It provides examples of the hydration and dehydration of cobalt(II) chloride and copper(II) sulfate. The document also discusses factors that affect equilibrium positions such as concentration, temperature, pressure, and catalysts. Finally, it summarizes the Haber process for producing ammonia and the contact process for producing sulfuric acid. Both processes use typical conditions of high pressure and temperature with a catalyst to favor production of the desired products.