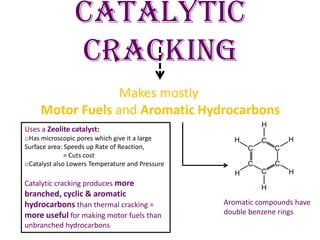
Thermal cracking and catalytic cracking are two types of hydrocarbon cracking used to produce lighter fractions from heavier fractions. Thermal cracking uses high temperatures and pressures to produce alkenes and alkanes, while catalytic cracking uses lower temperatures and a zeolite catalyst to produce motor fuels and aromatic hydrocarbons. Both cracking methods allow the production of lighter, more valuable fractions in higher demand to meet consumer needs.