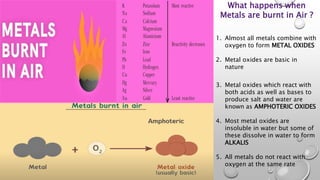
This document discusses the chemical properties of metals when reacted with air, water, acids, and other metal salt solutions. It explains that most metals form metal oxides when burned in air, which are often basic and can react with acids and bases. It also details that metals react with water to produce metal oxides and hydrogen gas, and react with acids to produce salts and hydrogen gas. Finally, it introduces displacement reactions where more reactive metals can displace less reactive metals from their salt solutions.