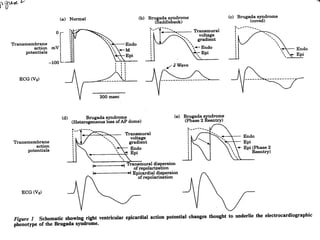
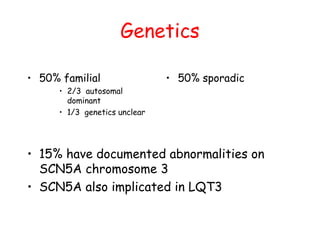
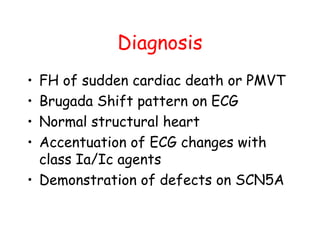
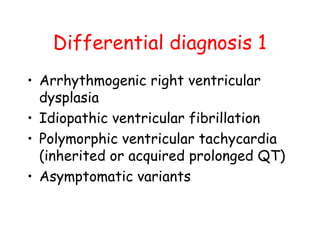
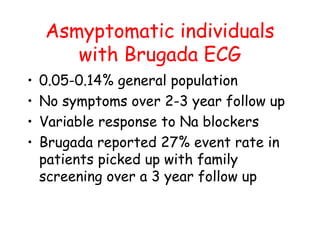
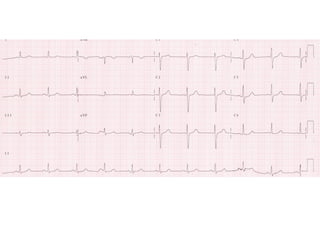
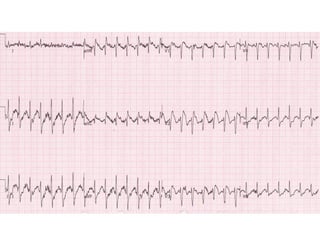
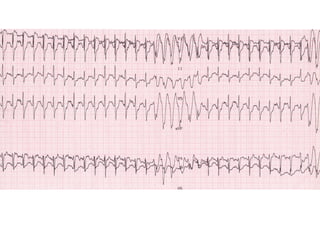
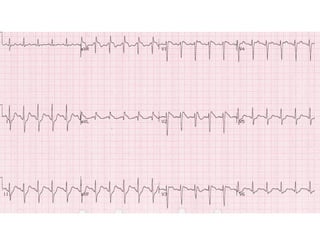
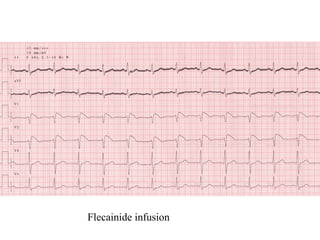
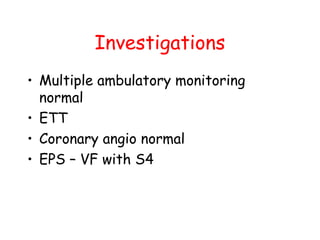
Brugada Syndrome is a genetic condition characterized by abnormal ECG patterns and risk of sudden cardiac death. It is caused by mutations in the SCN5A gene which result in arrhythmogenesis. Key features include ST segment elevation in leads V1-V3, increased risk in males, and episodes of polymorphic ventricular tachycardia or ventricular fibrillation. Diagnosis involves family history, ECG findings, and provocation of ECG changes with sodium channel blockers. Treatment involves lifestyle modifications and implantation of an ICD for high-risk individuals.