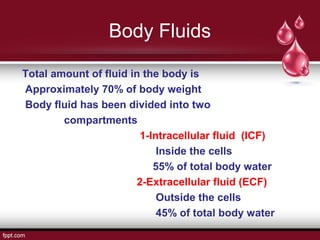
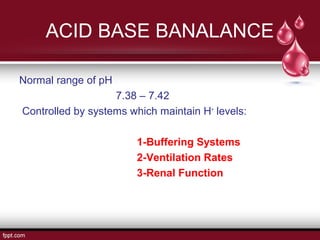
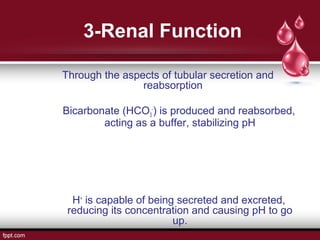
This document presents information about body fluid and acid-base balance. It discusses the composition and compartments of body fluids, including intracellular fluid, extracellular fluid, plasma, lymph, cerebrospinal fluid, amniotic fluid, milk, and tears. It also covers acid-base balance, noting the normal pH range is maintained by buffering systems, ventilation rates, and renal function. When there is too much or too little hydrogen ions, acid-base imbalances like acidosis or alkalosis can occur, which the respiratory and urinary systems work to compensate for and maintain pH homeostasis.