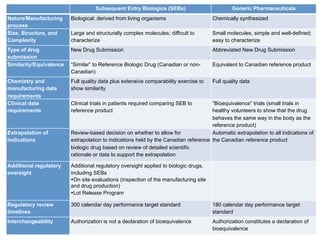
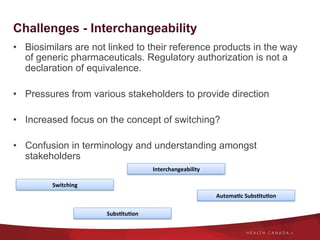
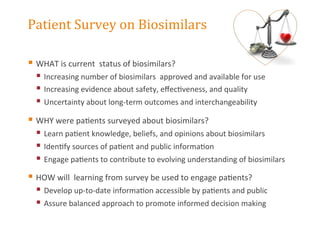
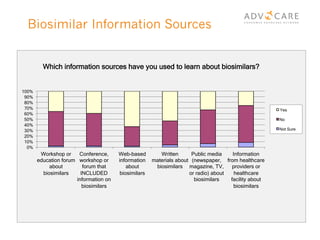
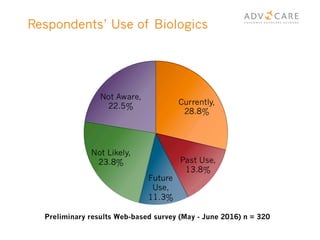
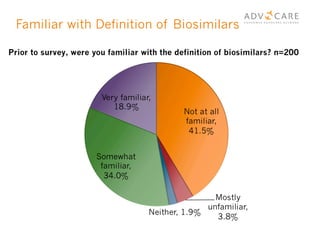
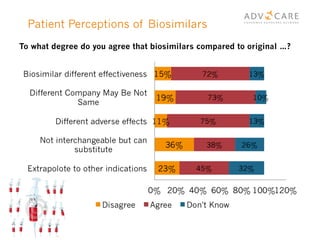
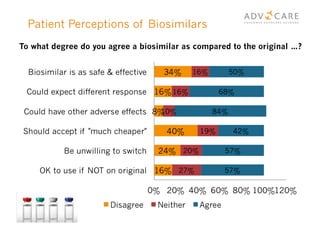
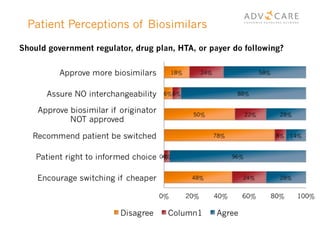
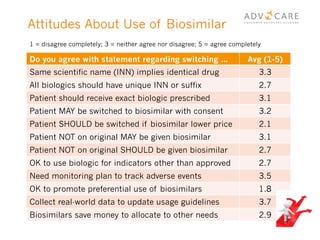
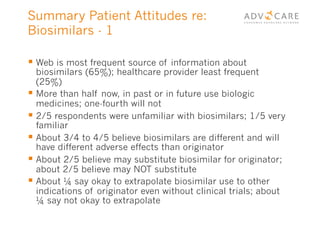
The document discusses the regulatory landscape for biosimilars in Canada, emphasizing their differences from generic drugs and the anticipated increase in their availability as patents expire. It outlines challenges related to interchangeability, patient perceptions, and the regulatory framework, including the need for comprehensive patient education on the safety and efficacy of biosimilars. The findings from a patient survey indicate varying levels of familiarity and confidence regarding biosimilars, with a significant need for transparent information and monitoring of outcomes.