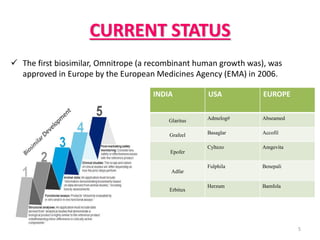
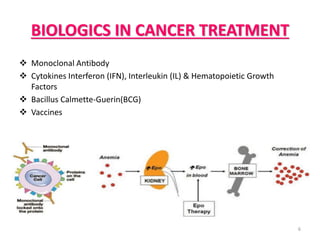
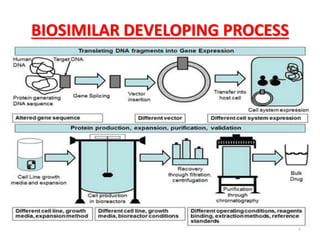
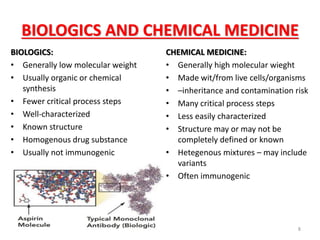
This document discusses biosimilars, which are biologic products that are highly similar to approved biologic reference products. It provides background on biosimilars, including their development process, advantages, limitations, and future outlook. The development process involves producing a cell line containing the gene for the desired protein, growing cells to produce the protein, purifying the protein, and preparing it for patient use. Biosimilars offer cost savings over biologics but have concerns around immunogenicity and long-term effects when switching between products. The global biosimilar market is expected to grow significantly as biologic patents expire and more companies develop biosimilar versions of treatments.